Details of the Drug
General Information of Drug (ID: DMPJKRL)
| Drug Name |
BITHIONOL
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
bithionol; 97-18-7; Actamer; Bithin; 2,2'-Thiobis(4,6-dichlorophenol); Lorothidol; Bitionol; Bithionol sulfide; Bisoxyphen; Bidiphen; Lorothiodol; Bitin; Nobacter; Bithionolate; Neopellis; Vancide BL; Usaf B-22; Bithional; Bithionolum; 2-Hydroxy-3,5-dichlorophenyl sulfide; TKhsd; Bis(2-hydroxy-3,5-dichlorophenyl) sulfide; Bis(3,5-dichloro-2-hydroxyphenyl) sulfide; 2,2'-sulfanediylbis(4,6-dichlorophenol); Caswell No. 852; Bitionol [INN-Spanish]; XL 7; Bithionolum [INN-Latin]; 2,2'-Dihydroxy-3,3',5,5'-tetrachlorodiphenyl sulfide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
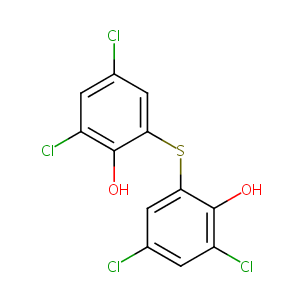 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 356 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Trematode infection | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 1F8Y | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
