| Drug Name |
SCH-442416
|
| Synonyms |
316173-57-6; SCH 442416; SCH-442416; UNII-ZMC4G1W59S; 2-(Furan-2-yl)-7-(3-(4-methoxyphenyl)propyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine; SCH442416; ZMC4G1W59S; CHEMBL136689; SCH-442,416; 2-(Furan-2-yl)-7-(3-(4-methoxyphenyl)propyl)-7H-pyrazolo-[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine; SCHEMBL981184; GTPL3283; CTK8B9468; CHEBI:92814; DTXSID70443263; MolPort-023-276-442; ZINC602847; HMS3269G07; BCP01942; MFCD08703126; BDBM50094037; ANW-62570; AKOS016003924; SCH442,416; NCGC00159575-01; AJ-23684; AC-27414
|
| Drug Type |
Small molecular drug
|
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
389.4 |
|
| Logarithm of the Partition Coefficient (xlogp) |
2.7 |
| Rotatable Bond Count (rotbonds) |
6 |
| Hydrogen Bond Donor Count (hbonddonor) |
1 |
| Hydrogen Bond Acceptor Count (hbondacc) |
7 |
| Chemical Identifiers |
- Formula
- C20H19N7O2
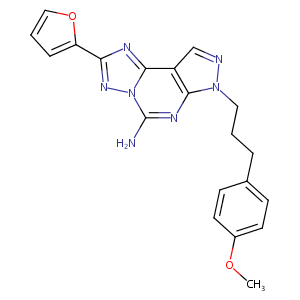
- IUPAC Name
4-(furan-2-yl)-10-[3-(4-methoxyphenyl)propyl]-3,5,6,8,10,11-hexazatricyclo[7.3.0.02,6]dodeca-1(9),2,4,7,11-pentaen-7-amine - Canonical SMILES
-
COC1=CC=C(C=C1)CCCN2C3=C(C=N2)C4=NC(=NN4C(=N3)N)C5=CC=CO5
- InChI
-
InChI=1S/C20H19N7O2/c1-28-14-8-6-13(7-9-14)4-2-10-26-18-15(12-22-26)19-23-17(16-5-3-11-29-16)25-27(19)20(21)24-18/h3,5-9,11-12H,2,4,10H2,1H3,(H2,21,24)
- InChIKey
-
AEULVFLPCJOBCE-UHFFFAOYSA-N
|
| Cross-matching ID |
- PubChem CID
- 10668061
- ChEBI ID
-
- CAS Number
-
- TTD ID
- D02YQO
|
|
|
|
|
|
|
|

