Details of the Drug
General Information of Drug (ID: DMQ2SNL)
| Drug Name |
2-(carboxymethylamino)-2-oxoacetic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
N-Oxalylglycine; 5262-39-5; Oxalylglycine; Oxaloglycine; 2-((Carboxymethyl)amino)-2-oxoacetic acid; UNII-VVW38EB8YS; N-(carboxycarbonyl)glycine; N-OXALYOLGLYCINE; VVW38EB8YS; 2-oxo-3-azaglutaric acid; CHEMBL90852; Glycine, N-(carboxycarbonyl)-; CHEBI:44482; OGA; C4H5NO5; 4idz; 4nrp; 2xml; 3hqr; N-oxalyl glycine, 1a; 2oq6; 1h2k; AC1MIVD0; Glycine,N-(carboxycarbonyl)-; SCHEMBL435820; BDBM26106; CTK8F0807; DTXSID20200601; MolPort-000-141-021; ZINC1534133; N-Oxalylglycine, > 4958AE
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
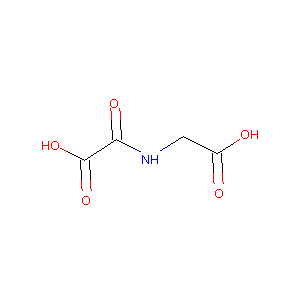 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 147.09 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
References
