Details of the Drug
General Information of Drug (ID: DMQ3LMF)
| Drug Name |
Ethyl 4-(2-oxo-2H-chromene-3-carboxamido)benzoate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
111947-24-1; ethyl 4-{[(2-oxo-2H-chromen-3-yl)carbonyl]amino}benzoate; AC1LHTSN; CBMicro_028869; 3-carboxamido coumarin, 15; Oprea1_481544; ethyl 4-(2-oxo-2H-chromene-3-carboxamido)benzoate; CHEMBL470419; BDBM29165; MolPort-000-375-263; MISJYZJMFQOBPG-UHFFFAOYSA-N; ZINC434162; STK401564; AKOS001279407; MCULE-7936571021; LS-38062; BIM-0028988.P001; ST4083371; ethyl 4-(2-oxo-2H-chromene-3-amido)benzoate; ethyl 4-[(2-oxochromen-3-yl)carbonylamino]benzoate; ethyl 4-[(2-oxochromene-3-carbonyl)amino]benzoate; Z30248586; F1006-0090
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
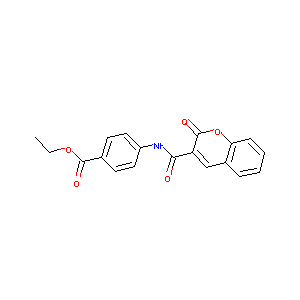 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 337.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
