Details of the Drug
General Information of Drug (ID: DMQ7UL8)
| Drug Name |
Camostat
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Camostat; 59721-28-7; camostate; Camostat [INN]; Camostatum [INN-Latin]; UNII-0FD207WKDU; CCRIS 7219; 4-(2-(2-(Dimethylamino)-2-oxoethoxy)-2-oxoethyl)phenyl 4-guanidinobenzoate; 0FD207WKDU; C20H22N4O5; Camostat (INN); Foipan; FOY 305; FOY-305; Dimethylcarbamoylmethyl 4-(4-guqnidinobenzoyloxy)phenylacetat; foypan; Camostatum; CHEMBL85164; camostate-mesilate; [4-[2-[2-(dimethylamino)-2-oxoethoxy]-2-oxoethyl]phenyl] 4-(diaminomethylideneamino)benzoate; p-Guanidinobenzoic acid, ester with (p-hydroxyphenyl)acetic acid, ester with N
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
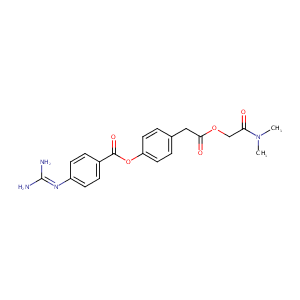 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 398.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
