Details of the Drug
General Information of Drug (ID: DMQ8HOJ)
| Drug Name |
lumateperone tosylate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
lumateperone Tosylate; lumateperone (Tosylate); 1187020-80-9; UNII-JIE88N006O; ITI-007 tosylate; JIE88N006O; 1187020-80-9 (tosylate); ITI007; ITI 007; ITI-007; Lumateperone tosylate (USAN); Lumateperone tosylate [USAN]; CHEMBL3233142; 1-(4-fluorophenyl)-4-[(6bR,10aS)-3-methyl-2,3,6b,9,10,10a-hexahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalin-8(7H)-yl]butan-1-one toluenesulfonic acid; Caplyta (TN); SCHEMBL1769664; CS-5540; HY-19733; D11170; Q27281520; 1-(4-Fluoro-phenyl)-4-((6bR,10aS)-3-methyl-2,3,6b,9,10,10a-hexahydro-1H,7H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalin-8-yl)-butan-1-one tosylate salt
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
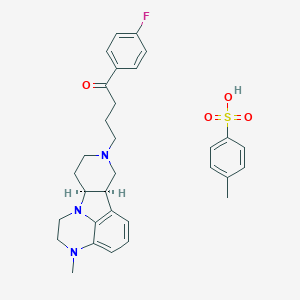 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 |
Molecular Weight | 565.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Schizophrenia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A20 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
