Details of the Drug
General Information of Drug (ID: DMQCXSG)
| Drug Name |
Avexitide
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Avexitide; Avexitide [USAN]; Exendin (9-39); Exendin 9-39; Exendin(9-39)amide; Exendin-(9-39); 5313W10MYT; UNII-5313W10MYT; Exendin 3 (heloderma horridum), 1-de-L-histidine-2-de-L-serine-3-de-L-aspartic acid-4-deglycine-5-de-L-threonine-6-de-L-phenylalanine-7-de-L-threonine-8-de-L-serine-; Exendin-3 (9-39) amide; exendin(9-39); AVEXITIDE [INN]; Exendin (9-39) amide; GTPL1138; EXENDIN (9-39) [MI]; DB14806; 9-39-EXENDIN 4 (HELODERMA SUSPECTUM); ASP-LEU-SER-LYS-GLN-MET-GLU-GLU-GLU-ALA-VAL-ARG-LEU-PHE-LLE-GLU-TRP-LEU-LYS-ASN-GIY-GIY-PRO-SER-SER-GIY-ALA-PRO-PRO-PRO-SER-NH2; L-SERINAMIDE, L-.ALPHA.-ASPARTYL-L-LEUCYL-L-SERYL-L-LYSYL-L-GLUTAMINYL-L-METHIONYL-L-.ALPHA.-GLUTAMYL-L-.ALPHA.-GLUTAMYL-L-.ALPHA.-GLUTAMYL-L-ALANYL-L-VALYL-L-ARGINYL-L-LEUCYL-L-PHENYLALANYL-L-ISOLEUCYL-L-.ALPHA.-GLUTAMYL-L-TRYPTOPHYL-L-LEUCYL-L-LYSYL-L-ASPARAGINYLGLYCYLGLYCYL-L-PROLYL-L-SERYL-L-SERYLGLYCYL-L-ALANYL-L-PROLYL-L-PROLYL-L-PROLYL-
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Peptide
|
||||||||||||||||||||||||||
| Structure |
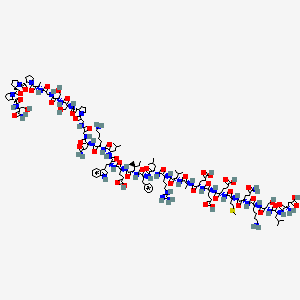 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Congenital hyperinsulinism | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A4Y | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
