Details of the Drug
General Information of Drug (ID: DMRDZ4X)
| Drug Name |
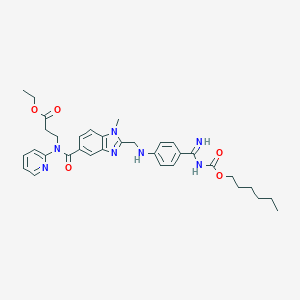
Dabigatran etexilate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
BIBR-1048; 211915-06-9; Dabigatran etexilate mesilate; ethyl N-[(2-{[(4-{N'-[(hexyloxy)carbonyl]carbamimidoyl}phenyl)amino]methyl}-1-methyl-1H-benzimidazol-5-yl)carbonyl]-N-pyridin-2-yl-beta-alaninate; Dabigatran etexilate methanesulfonate; BIBR-1048 (Dabigatran); UNII-2E18WX195X; BIBR1048; BIBR-1048-BS-RS1; CHEBI:70746; 2E18WX195X; cc-72; DSSTox_CID_31470; DSSTox_RID_97355; DSSTox_GSID_57681; SCHEMBL505829; DTXSID4057681; Dabigatran etexilate (USAN/INN); MolPort-016-633-287; EBD35035; Tox21_113924; BDBM504
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 627.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 18 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
|---|---|---|---|---|---|
| 2 | FDA Approved Drug Products: PRADAXA (dabigatran) oral pellets | ||||
| 3 | Szultka M, Krzeminski R, Jackowski M, Buszewski B: Identification of In Vitro Metabolites of Amoxicillin in Human Liver Microsomes by LC-ESI/MS. Chromatographia. 2014;77:1027-1035. doi: 10.1007/s10337-014-2648-2. Epub 2014 Mar 22. | ||||
| 4 | Dabigatran acylglucuronide, the major human metabolite of dabigatran: in vitro formation, stability, and pharmacological activity. Drug Metab Dispos. 2010 Sep;38(9):1567-75. | ||||
| 5 | Pharmacogenomics of novel direct oral anticoagulants: newly identified genes and genetic variants. J Pers Med. 2019 Jan 17;9(1). pii: E7. | ||||
