Details of the Drug
General Information of Drug (ID: DMRGZ16)
| Drug Name |
Sulfamethazine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Azolmetazin; Cremomethazine; Diazil; Dimezathine; Intradine; Kelametazine; Mermeth; Neasina; Neazina; Pirmazin; Primazin; Solfadimidina; Spanbolet; Sulfadimerazine; Sulfadimesin; Sulfadimesine; Sulfadimethyldiazine; Sulfadimethylpyrimidine; Sulfadimezin; Sulfadimezine; Sulfadimezinum; Sulfadimidin; Sulfadimidina; Sulfadimidine; Sulfadimidinum; Sulfadine; Sulfametazina; Sulfametazyny; Sulfamethiazine; Sulfamezathine; Sulfodimesin; Sulfodimezine; Sulphadimethylpyrimidine; Sulphadimidine; Sulphamethasine; Sulphamethazine; Sulphamezathine; Sulphamidine; Sulphodimezine; Superseptil; Superseptyl; Vertolan; Calfspan Tablets; Sa III; Solfadimidina [DCIT]; SulfaSURE SR Bolus; Sulfadimidine solution; Sulfametazina[Italian]; Sulfametazyny [Polish]; Sulfamethazine solution; Sulka S Boluses; BN 2409; HC210279; A-502; Diazil (the sulfanilamide); Dimidin-R; Hava-Span; Sulfa-Isodimerazine; Sulfadimidina [INN-Spanish]; Sulfadimidine (INN); Sulfadimidine [INN:BAN]; Sulfadimidinum [INN-Latin]; Sulfamethazine (USP); Sulfamezathine (TN); N-(4,6-Dimethyl-2-pyrimidyl)sulfanilamide; N(1)-(4,6-Dimethyl-2-pyrimidinyl)sulfanilamide; N(1)-(4,6-Dimethyl-2-pyrimidyl)sulfanilamide; N(Sup1)-(4,6-Dimethyl-2-pyrimidinyl)sulfanilamide; N(Sup1)-(4,6-Dimethyl-2-pyrimidyl)sulfanilamide; N(sup 1)-(4,6-Dimethyl-2-pyrimidinyl)sulfanilamide; N(sup 1)-(4,6-Dimethyl-2-pyrimidyl)sulfanilamide; N(sup1)-(2,6-Dimethylpyrimid-4-yl)sulfanilamide; [(4-Aminophenyl)sulfonyl](4,6-dimethylpyrimidin-2-yl)amine; (p-Aminobenzolsulfonyl)-2-amino-4,6-dimethylpyrimidin; (p-Aminobenzolsulfonyl)-2-amino-4,6-dimethylpyrimidin [German]; 2-(4-Aminobenzenesulfonamido)-4,6-dimethylpyrimidine; 2-(p-Aminobenzenesulfonamido)-4,6-dimethylpyrimidine; 2-Sulfanilamido-4,6-dimethylpyrimidine; 4,6-Dimethyl-2-sulfanilamidopyrimidine; 4,6-Dimethylsulfadiazine; 4-Amino-N-(2,6-dimethyl-4-pyrimidinyl)benzenesulfonamide; 4-Amino-N-(4,6-dimethyl-2-pyrimidinyl)benzenesulfonamide; 4-Amino-N-(4,6-dimethyl-2-pyrimidyl)benzenesulfonamide; 4-Amino-N-(4,6-dimethyl-pyrimidin-2-yl)-benzenesulfonamide; 4-Amino-N-[4,6-dimethyl-2-pyrimidinyl]-benzenesulfonamide; 4-amino-N-(4,6-dimethylpyrimidin-2-yl)benzene-1-sulfonamide; 4-amino-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide; 6-(4'-Aminobenzol-sulfonamido)-2,4-dimethylpyrimidin; 6-(4'-Aminobenzol-sulfonamido)-2,4-dimethylpyrimidin [German]
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiinfective Agents
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
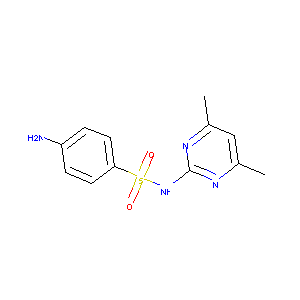 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 278.33 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
