| Drug Name |
ARISTEROMYCIN
|
| Synonyms |
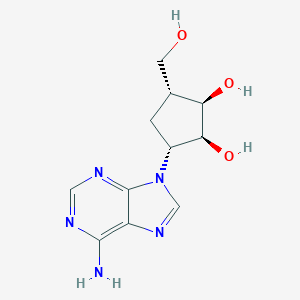
19186-33-5; 3-(6-aminopurin-9-yl)-5-(hydroxymethyl)cyclopentane-1,2-diol; NSC613806; NSC615828; NSC613807; NSC103526; Carbocyclic Lyxo-A; Carbocyclic Xylo-A; AC1Q4VH0; Cyclaradine Carbocyclic Ara A; SCHEMBL9820408; AC1L1D83; CTK4E0829; 3-(6-amino-9h-purin-9-yl)-5-(hydroxymethyl)cyclopentane-1,2-diol; NSC-613807; NSC-613806; NSC-615828; NSC-103526; J-012404; 1,2-Cyclopentanediol,5-(6-amino-9H-purin-9-yl)-3-(hydroxymethyl)-, (1S,2R,3R,5R)-
|
| Drug Type |
Small molecular drug
|
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
265.27 |
|
| Logarithm of the Partition Coefficient (xlogp) |
-0.5 |
| Rotatable Bond Count (rotbonds) |
2 |
| Hydrogen Bond Donor Count (hbonddonor) |
4 |
| Hydrogen Bond Acceptor Count (hbondacc) |
7 |
| Chemical Identifiers |
- Formula
- C11H15N5O3
- IUPAC Name
(1R,2S,3R,5R)-3-(6-aminopurin-9-yl)-5-(hydroxymethyl)cyclopentane-1,2-diol - Canonical SMILES
-
C1[C@@H]([C@H]([C@H]([C@@H]1N2C=NC3=C(N=CN=C32)N)O)O)CO
- InChI
-
InChI=1S/C11H15N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h3-6,8-9,17-19H,1-2H2,(H2,12,13,14)/t5-,6-,8-,9+/m1/s1
- InChIKey
-
UGRNVLGKAGREKS-GCXDCGAKSA-N
|
| Cross-matching ID |
- PubChem CID
- 65269
- CAS Number
-
- TTD ID
- D0I7BL
|
|
|
|
|
|
|
|

