Details of the Drug
General Information of Drug (ID: DMSB8WQ)
| Drug Name |
6,11-dihydro-5H-benzo[a]carbazole
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
6,11-dihydro-5H-benzo[a]carbazole; 21064-49-3; 5H-Benzo[a]carbazole, 6,11-dihydro-; 1,2,7-trihydrobenzo[a]4aH-carbazole; ST076800; 5,11-Dihydro-6H-benzo[a]carbazole; 5,11-DIHYDRO-6H-BENZO(A)CARBAZOLE; NSC168810; AC1L6RUQ; Maybridge1_006237; AC1Q1H7M; CHEMBL239937; Aza-heterocyclic Derivative, 7; SCHEMBL12126575; HMS559D11; CTK0J8060; BDBM19186; DTXSID40305026; MolPort-000-737-601; ZX-AN022290; ALBB-023776; ZINC1023865; CCG-20433; STK874208; BBL028411; 5,6-Dihydro-11H-benzo[a]carbazole; AKOS000547063; MCULE-4710620349; NSC-168810; RH 00
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
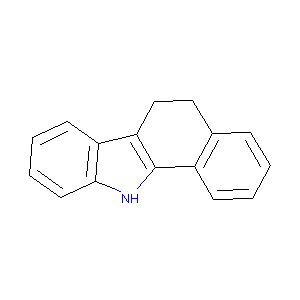 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 219.28 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 0 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
