Details of the Drug
General Information of Drug (ID: DMSFQ8I)
| Drug Name |
Meclocycline
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Meclociclina; Meclocyclinum; Meclosorb; Samil; Meclociclina [Italian]; GS 2989; GS-2989; Meclociclina [INN-Spanish]; Meclocycline(USAN); Meclocyclinum [INN-Latin]; Meclocycline (USAN/INN); Meclocycline [USAN:INN:BAN]; (2E,4S,4aR,5S,5aR,12aS)-2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methylidene-4,4a,5,5a-tetrahydrotetracene-1,3,12-trione; (2E,4aR,5S,5aS,12aS)-2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methylidene-4,4a,5,5a-tetrahydrotetracene-1,3,12-trione; (2Z)-2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methylidene-4,4a,5,5a-tetrahydrotetracene-1,3,12-trione; (2Z,4S,4aR,5S,5aR,12aS)-2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methylidene-4,4a,5,5a-tetrahydrotetracene-1,3,12-trione; 2-Naphthacenecarboxamide, 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-, [4S-(4.alpha.,4a.alpha.,5.alpha.,5a.alpha.,12a.alpha.)]-(9CI); 2-Naphthacenecarboxamide, 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-, [4S-(4.alpha.,4aal; 2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methylidene-4,4a,5,5a-tetrahydrotetracene-1,3,12-trione; 7-Chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-2-naphthacenecarboxamide; 7-Chloro-6-methylene-5-oxytetracycline; 7-Cloro-6-metilene-5-ossitetraciclina; 7-Cloro-6-metilene-5-ossitetraciclina [Italian]; 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-2-naphthacene carboxamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
||||||||||||||||||||||
| Affected Organisms |
Gram negative and gram positive bacteria
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
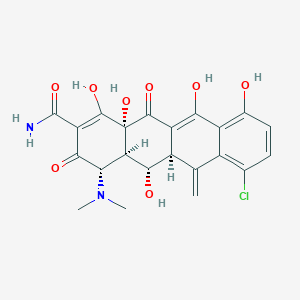 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 476.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
