Details of the Drug
General Information of Drug (ID: DMSJGQE)
| Drug Name |
3-(3-Carboxy-propionylamino)-benzoic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL296834; 3-(3-carboxypropanamido)benzoic acid; 3-[(3-carboxypropanoyl)amino]benzoic acid; 259222-20-3; IFLab1_001254; 3-(3-carboxypropanoylamino)benzoic acid; 3-(3-Carboxy-propionylamino)-benzoic acid; AC1LFPO3; Oprea1_683242; Oprea1_208346; CTK0J3689; DTXSID30353899; MolPort-000-385-127; HMS1415I22; ZINC216961; STK032065; BDBM50127981; AKOS000112854; MCULE-6483245282; IDI1_009121; ST50036495; 3-(4-Oxo-4-hydroxybutyrylamino)benzoic acid; 3-[(4-hydroxy-4-oxobutanoyl)amino]benzoic acid; SR-01000005669; Benzoic acid, 3-[(3-carbo
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
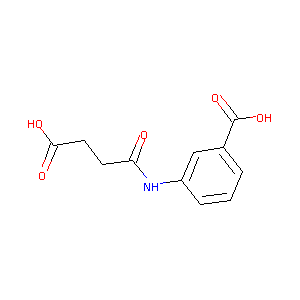 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 237.21 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
