Details of the Drug
General Information of Drug (ID: DMSQLZJ)
| Drug Name |
Nifurtimox
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
NIFURTIMOX; Lampit; Bayer 2502; 23256-30-6; BAY 2502; Nifurtimox [INN:BAN]; Nifurtimoxum [INN-Latin]; CCRIS 2201; EINECS 245-531-0; CHEBI:7566; C10H13N3O5S; 4-Thiomorpholinamine, 3-methyl-N-((5-nitro-2-furanyl)methylene)-, 1,1-dioxide; 4-((5-Nitrofurfurylidene)amino)-3-methylthiomorpholine-1,1-dioxide; 3-Methyl-N-[(5-nitro-2-furanyl)methylene]-4-thiomorpholinamine 1,1-dioxide; 3-Methyl-4-(5'-nitrofurylidene-amino)-tetrahydro-4H-1,4-thiazine-1,1-dioxide
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Trypanosoma cruzi
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
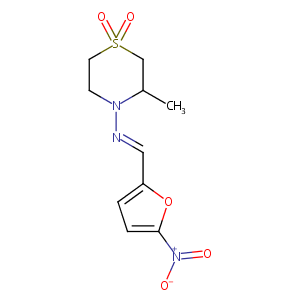 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 287.29 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.3 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | ||||
|---|---|---|---|---|---|
| 2 | ClinicalTrials.gov (NCT00906880) Clinical Study to Assess the Tolerability, Feasibility and Effectiveness of Nifurtimox and Eflornithine (NECT) for the Treatment of Trypanosoma Brucei Gambiense HumanAfrican Trypanosomiasis (HAT) in the Meningo-encephalitic Phase. U.S. National Institutes of Health. | ||||
| 3 | FDA Approved Products: Lampit (nifurtimox) oral tablets | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Gonzalez-Martin G, Thambo S, Paulos C, Vasquez I, Paredes J: The pharmacokinetics of nifurtimox in chronic renal failure. Eur J Clin Pharmacol. 1992;42(6):671-3. doi: 10.1007/BF00265935. | ||||
| 6 | Hoffmann M, Kumar G, Schafer P, Cedzik D, Capone L, Fong KL, Gu Z, Heller D, Feng H, Surapaneni S, Laskin O, Wu A: Disposition, metabolism and mass balance of [(14)C]apremilast following oral administration. Xenobiotica. 2011 Dec;41(12):1063-75. doi: 10.3109/00498254.2011.604745. Epub 2011 Aug 23. | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | The transport of nifurtimox, an anti-trypanosomal drug, in an in vitro model of the human blood-brain barrier: evidence for involvement of breast cancer resistance protein. Brain Res. 2012 Feb 3;1436:111-21. | ||||
