Details of the Drug
General Information of Drug (ID: DMSV2WZ)
| Drug Name |
Chlorotrianisene
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Anisene; Chloortrianisestrol; Chlorestrolo; Chlorotrianisenum; Chlorotrianisestrol; Chlorotrianisine; Chlorotrianizen; Chlorotrisin; Chlortrianisen; Chlortrianisene; Chlortrianisenum; Chlortrianisestrol; Chlortrianisoestrolum; Chlortrianizen; Clorestrolo; Clorotrianisene; Clorotrianiseno; Clorotrisin; Hormonisene; Khlortrianizen; Merbentul; Metace; Rianil; TACE; Triagen; Trianisestrol; Chlorotrianisene [Nonsteroidal oestrogens]; Clorotrianisene [DCIT]; Chlorotrianisene (INN); Chlorotrianisene [BAN:INN]; Chlorotrianisene [INN:BAN]; Chlorotrianisenum [INN-Latin]; Clorotrianiseno [INN-Spanish]; TACE (TN); Tace (pharmaceutical); Tace-fn; Chlorotris(p-methoxyphenyl)ethylene; Tri-p-anisylchloroethylene; Tris(p-methoxyphenyl)chloroethylene; 1,1',1''-(1-Chloro-1-ethenyl-2-ylidene)-tris(4-methoxybenzene); 1,1',1''-(2-chloroethene-1,1,2-triyl)tris(4-methoxybenzene); 1,1',1''-(2-chloroethene-1,1,2-triyl)tris[4-(methyloxy)benzene]; 1-[1-chloro-2,2-bis(4-methoxyphenyl)ethenyl]-4-methoxybenzene
|
|||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
|||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||
| Structure |
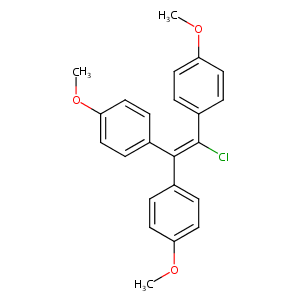 |
|||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 380.9 | ||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.4 | |||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | |||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||||||||||||||||||||||
| ADMET Property | ||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
