Details of the Drug
General Information of Drug (ID: DMSYFM8)
| Drug Name |
Cysteamine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Becaptan; Cisteamina; Cystavision; Cysteamide; Cysteamin; Cysteinamine; Decarboxycysteine; Ethanethiolamine; Lambraten; Lambratene; MEA; Mecramine; Mercamin; Mercamine; Mercaptamin; Mercaptamina; Mercaptamine; Mercaptaminum; Mercaptoethylamine; Merkamin; Riacon; Thioethanolamine; Aminoethyl mercaptan; Cisteamina [Italian]; Cysteamine bitartate; L 1573; WR 347; Beta-Aminoethanethiol; Beta-Aminoethylthiol; Beta-MEA; Beta-Mercaptoethylamine; C-9500; Cysteamine (USAN); Cysteamine [USAN:BAN]; L-1573; MEA (mercaptan); Mercaptamina [INN-Spanish]; Mercaptamine (INN); Mercaptaminum [INN-Latin]; (2-Mercaptoethyl)amine; (Mercaptoethyl)ammonium toluene-p-sulphonate; 1-Amino-2-mercaptoethylamine; 2-AMINO-ETHANETHIOL; 2-Amino-1-ethanethiol; 2-Aminoethanethiol; 2-Aminoethyl mercaptan; 2-Mercaptoethanamine; 2-Mercaptoethylamine; 2-Mercaptoethylamine, polymer-bound; 641022_ALDRICH
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Nephropathic cystinosis therapy
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
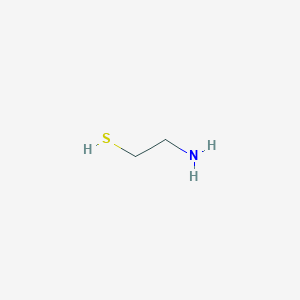 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 77.15 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | An Open-Label Investigation of the Pharmacokinetics and Tolerability of Oral Cysteamine in Adults with Cystic Fibrosis. Clin Drug Investig. 2016 Aug;36(8):605-12. doi: 10.1007/s40261-016-0405-z. | ||||
| 3 | FDA approved products: PROCYSBI (cysteamine bitartrate) delayed-release oral capsules | ||||
| 4 | Somatostatin, Alzheimer's disease and cognition: an old story coming of age Prog Neurobiol. 2009 Oct;89(2):153-61. | ||||
| 5 | Thiols as peroxidase substrates. Free Radic Biol Med. 1993 Feb;14(2):167-75. | ||||
| 6 | Cysteamine suppresses invasion, metastasis and prolongs survival by inhibiting matrix metalloproteinases in a mouse model of human pancreatic cancer. PLoS One. 2012;7(4):e34437. doi: 10.1371/journal.pone.0034437. Epub 2012 Apr 20. | ||||
