Details of the Drug
General Information of Drug (ID: DMTGNWU)
| Drug Name |
Tofisopam
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
tofisopam; 22345-47-7; Grandaxin; Seriel; 1-(3,4-Dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5H-2,3-benzodiazepine; Egyt 341; Tofisopamum; 5H-2,3-Benzodiazepine, 1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-; tofizopam; NCGC00165912-02; 1-(3,4-Dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5H-benzo[d][1,2]diazepine; benzodiazepine; EGYT 341; Grandaxin; Nodeprine; Seriel; Tofisopamum [INN-Latin]; 7,8-Dimethoxy-1-(3,4-dimethoxyphenyl)-5-ethyl-4-methyl-5H-2,3-benzodiazepine; Tofisopam [INN:DCF:JAN]; EINECS 244-922-3; CCRIS 8738; Emandaxin (TN); DSSTox_CID_3681; Tofisopam (JP17/INN); DSSTox_RID_77145; DSSTox_GSID_23681; SCHEMBL43522; Tofisopam (patented in japan); CHEMBL404216; SCHEMBL8086894; DTXSID3023681; CHEBI:32241; BCP09600; Tox21_112269; MFCD00823171; Tofisopam, >=98% (HPLC), solid; AKOS025401672; DB08811; QC-1212; NCGC00165912-01; NCGC00165912-03; AC-24288; LS-14948; CAS-22345-47-7; DB-045872; FT-0638212; X6663; D01254; 345T477; Q945537; Q-201839; BRD-A69095630-001-01-1; 1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7,8-dimethoxy-5H-2,3-benzodiazepine; 1-[3,4-bis(methyloxy)phenyl]-5-ethyl-4-methyl-7,8-bis(methyloxy)-5H-2,3-benzodiazepine; (1Z,3Z)-1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5H-benzo[d][1,2]diazepine
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
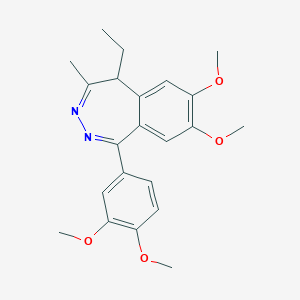 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 382.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.2 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hyperuricaemia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5C55.Y | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
