Details of the Drug
General Information of Drug (ID: DMTKSVO)
| Drug Name |
T0070907
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
2-Chloro-5-nitro-N-4-pyridinylbenzamide; 313516-66-4; 2-CHLORO-5-NITRO-N-4-PYRIDINYLBENZAMIDE; 2-chloro-5-nitro-N-(pyridin-4-yl)benzamide; T 0070907; T-0070907; 2-chloro-5-nitro-N-pyridin-4-ylbenzamide; 2-Chloro-5-nitro-N-(4-pyridyl)benzamide; CHEMBL510698; Benzamide, 2-chloro-5-nitro-N-4-pyridinyl-; 2-chloro-5-nitro-n-4-pyridinyl-benzamide; SR-01000392700; AC1MCROG; Oprea1_586106; ZINC3381; GTPL3444; SCHEMBL2128178; CTK6H1028; KS-00000MYU; CHEBI:92553; DTXSID30380504; MolPort-001-763-336; FRPJSHKMZHWJBE-UHFFFAOYSA-N; HMS3268J16; HMS3651P21; HMS3262J21; T 0070907
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
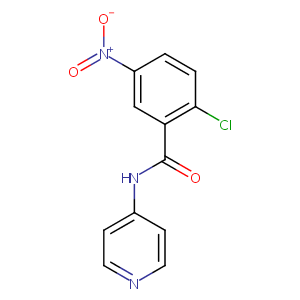 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 277.66 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
References
