Details of the Drug
General Information of Drug (ID: DMTLOU6)
| Drug Name |
CLEBOPRIDE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Clebopride; 55905-53-8; Cleboril; Clebopridum [INN-Latin]; Cleboprida [INN-Spanish]; 4-amino-N-(1-benzylpiperidin-4-yl)-5-chloro-2-methoxybenzamide; UNII-I0A84520Y9; LAS 9273; EINECS 259-885-9; C20H24ClN3O2; BRN 0493934; CHEMBL325109; I0A84520Y9; 4-Amino-N-(1-benzyl-4-piperidyl)-5-chloro-o-anisamide; clebopride malate; 4-Amino-5-chloro-2-methoxy-N-(1-benzyl-4-piperidyl)benzamide; Clebopridum; Cleboprida; N-(1'-Benzyl-4'-piperidyl)-2-methoxy-4-amino-5-chlorobenzamide; BENZAMIDE, 4-AMINO-5-CHLORO-2-METHOXY-N-(1-(PHENYLMETHYL)-4
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
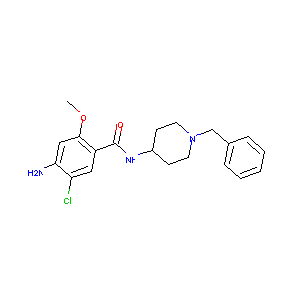 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 373.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
