Details of the Drug
General Information of Drug (ID: DMTX0PZ)
| Drug Name |
Dibekacin
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Debecacin; Dibekacin sulfate; Dibekacina; Dibekacina [INN-Spanish]; Dibekacine; Dibekacine [INN-French]; Dibekacinum; Dibekacinum [INN-Latin]; Dideoxykanamycin B; Kappati; Panamicin; dibekacin; 3',4'-Dideoxykanamycin B; 34493-98-6; 45ZFO9E525; BRN 1441606; CHEBI:37945; DKB; DKM; EINECS 252-064-6; O-3-Amino-3-deoxy-alpha-D-glucopyranosyl-(1-4)-O-(2,6-diamino-2,3,4,6-tetradeoxy-alpha-D-erythro-hexopyranosyl-(1-6))-2-deoxy-L-streptamine; UNII-45ZFO9E525
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Structure |
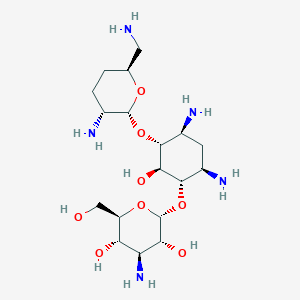 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 451.5 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -5.8 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 9 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 13 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
