Details of the Drug
General Information of Drug (ID: DMUVKX5)
| Drug Name |
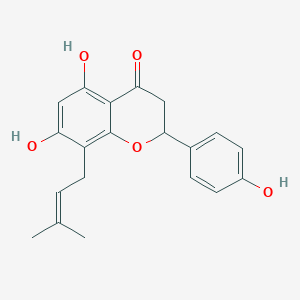
SOPHORAFLAVANONE B
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
8-Prenylnaringenin; 53846-50-7; Sophoraflavanone B; Flavaprenin; (-)-(2S)-8-dimethylallylnaringenin; (S)-8-dimethylallylnaringenin; YS04; CHEMBL376915; CHEBI:50207; (-)-(2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-8-(3-methylbut-2-enyl)chroman-4-one; (2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-8-(3-methylbut-2-en-1-yl)-2,3-dihydro-4H-chromen-4-one; (s)-8-prenylnaringenin; 8-dimethylallylnaringenin; AC1LA3DM; UNII-5L872SZR8X; 8-prenylnaringenin (8-PN); BIDD:ER0149; cid_509245; SCHEMBL1171435; 5L872SZR8X; ZINC39452; CTK5J8800; BDBM19460
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 340.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
