Details of the Drug
General Information of Drug (ID: DMV23GL)
| Drug Name |
LIDOFLAZINE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
LIDOFLAZINE; Ordiflazine; Corflazine; Clinium; Lidoflazinum; Lidoflazin; 3416-26-0; Klinium; Angex; Lidoflazina [Italian]; McN-JR 7904; UNII-J4ZHN3HBTE; Lidoflazinum [INN-Latin]; Lidoflazina [INN-Spanish]; Lidoflazine [USAN:INN:BAN]; C30H35F2N3O; J4ZHN3HBTE; EINECS 222-312-8; R 7904; BRN 0904339; CHEMBL92870; MCN-JR-7904; 4-(4,4-Bis(p-fluorophenyl)butyl)-1-piperazineaceto-2',6'-xylidide; 1-Piperazineacetamide, 4-(4,4-bis(4-fluorophenyl)butyl)-N-(2,6-dimethylphenyl)-; NCGC00016627-01; NCGC00016627-04; CAS-3416-26-0
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
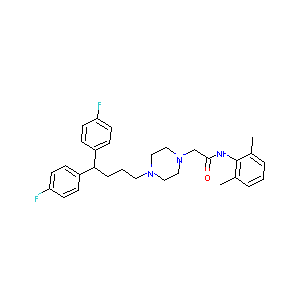 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 491.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
