Details of the Drug
General Information of Drug (ID: DMXMAJA)
| Drug Name |
Fosgonimeton
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Fosgonimeton; Fosgonimeton [INN]; Fosgonimeton [USAN]; UNII-H91OA9858J; H91OA9858J; 2093305-05-4; Fosgonimeton [USAN:INN]; ATH-1017; NDX-1017; WHO 11782; L-Isoleucinamide, O-(phosphono-kappaO)-N-(1-oxohexyl)-L-tyrosyl-N-(6-amino-6-oxohexyl)-,; ATH-1017 FREE ACID; NDX-1017 FREE ACID; CHEMBL5095419; AKOS040757261; HY-132814; CS-0204081; L-Isoleucinamide, O-(phosphono-kappaO)-N-(1-oxohexyl)-L-tyrosyl-N-(6-amino-6-oxohexyl)-; dihydrogen 4-[(2S)-3-({(2S,3S)-1-[(6-amino-6-oxohexyl)amino]-3-methyl-1-oxopentan-2-yl}amino)-2-hexanamido-3-oxopropyl]phenyl phosphate
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||||||
| Structure |
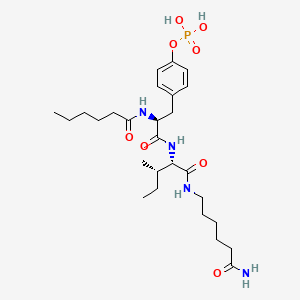 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
