Details of the Drug
General Information of Drug (ID: DMXYA5K)
| Drug Name |
Agomelatine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Thymanax; Valdoxan; Agomelatine [INN]; AGO 178; AGO178; S 20098; S20098; AGO-178; Agomelatine(INN); S-20098; Valdoxan (TN); Valdoxan, Melitor, Thymanax, Agomelatine; N-[2-(7-methoxynaphthalen-1-yl)ethyl]acetamide; N-(2-(7-Methoxy-1-naphthalenyl)ethyl)acetamide; N-(2-(7-Methoxynaphth-1-yl)ethyl)acetamide; N-(2-(7-methoxy-1-naphthyl)ethyl)acetamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
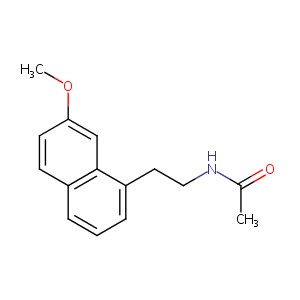 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 243.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 06 Mental, behavioural or neurodevelopmental disorder | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: 6A20 Schizophrenia | |||||||||||||||||||||||||||||
| The Studied Tissue | Pre-frontal cortex | |||||||||||||||||||||||||||||
| The Studied Disease | Major depressive disorder [ICD-11:6A20] | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
