Details of the Drug
General Information of Drug (ID: DMXYLTI)
| Drug Name |
CDP323
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Zaurategrast; 455264-31-0; (S)-3-(4-((2,7-Naphthyridin-1-yl)amino)phenyl)-2-((2-bromo-3-oxospiro[3.5]non-1-en-1-yl)amino)propanoic acid; UNII-06A0IC74I3; 06A0IC74I3; C26H25BrN4O3; Zaurategrast [INN]; SCHEMBL2976322; CTK8C0588; DTXSID90196547; MolPort-023-332-826; KS-00001DY0; ANW-64932; 6274AB; ZINC100041912; AKOS016005046; CS-0322; N-(2-Bromo-3-oxospiro[3.5]non-1-en-1-yl)-4-(2,7-naphthyridin-1-ylamino)-L-phenylalanine; NCGC00378753-01
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
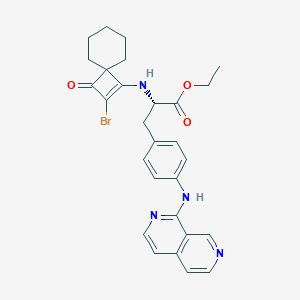 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 549.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.1 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
