| Drug Name |
Carbapenem
|
| Synonyms |
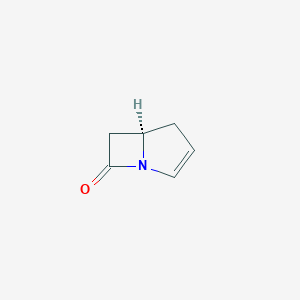
Carbapenem; Cis-carbapenem; (5R)-1-azabicyclo[3.2.0]hept-2-en-7-one; 2,3-didehydro-1-carbapenam; 67836-EP2275424A1; 67836-EP2295402A2; 67836-EP2305219A1; 67836-EP2308874A1; CHEBI:46765; SCHEMBL37243 |
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
109.13 |
|
| Logarithm of the Partition Coefficient (xlogp) |
0 |
| Rotatable Bond Count (rotbonds) |
0 |
| Hydrogen Bond Donor Count (hbonddonor) |
0 |
| Hydrogen Bond Acceptor Count (hbondacc) |
1 |
| Chemical Identifiers |
- Formula
- C6H7NO
- IUPAC Name
(5R)-1-azabicyclo[3.2.0]hept-2-en-7-one - Canonical SMILES
-
C1C=CN2C1CC2=O
- InChI
-
YZBQHRLRFGPBSL-RXMQYKEDSA-N
- InChIKey
-
1S/C6H7NO/c8-6-4-5-2-1-3-7(5)6/h1,3,5H,2,4H2/t5-/m1/s1
|
| Cross-matching ID |
- PubChem CID
- 13293131
- ChEBI ID
-
- INTEDE ID
- DR2678
|
|
|
|
|
|
|
|



