Details of the Drug
General Information of Drug (ID: DMY0KGJ)
| Drug Name |
Chlorprothixene
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Chloroprothixene; Chlorprothixen; Chlorprothixenum; Chlorprothixine; Chlorprotixen; Chlorprotixene; Chlorprotixine; Chlothixen; Clorprotisene; Clorprotixeno; Iaractan; Paxyl; Rentovet; Tactaran; Taractan; Tarasan; Tardan; Traquilan; Trictal; Truxal; Truxaletten; Truxil; Vetacalm; Clorprotisene [DCIT]; MK 184; N 714; N 714C; NCI56378; Alpha-Chlorprothixene; Chlorprothixenum [INN-Latin]; Cis-Chlorprothixene; Clorprotixeno [INN-Spanish]; N-714; Ro 4-0403; Taractan (TN); Truxal (TN); Ro-4-0403;Trans(E)-Chlorprothixen; Chlorprothixene (JAN/USAN/INN); Chlorprothixene [USAN:INN:BAN:JAN]; Cloxan, Taractan, Truxal, Chlorprothixene; Cis-2-Chloro-9-(3-dimethylaminopropylidene)thioxanthene; {3-[2-Chloro-thioxanthen-(9Z)-ylidene]-propyl}-dimethyl-amine; Thioxanthene-delta9,gamma-propylamine, 2-chloro-N,N-dimethyl-, (Z)-(8CI); (3E)-3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N-dimethylpropan-1-amine; (3E)-3-(2-chlorothioxanthen-9-ylidene)-N,N-dimethylpropan-1-amine; (3Z)-3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N-dimethylpropan-1-amine; (3Z)-3-(2-chlorothioxanthen-9-ylidene)-N,N-dimethylpropan-1-amine; (Z)-2-Chloro-9-(omega-dimethylaminopropylidene)thioxanthene; (Z)-2-Chloro-N,N-dimethylthioxanthene-.DELTA.(sup9),(sup.gamma.)-propylamine; (Z)-2-Chloro-N,N-dimethylthioxanthene-delta(sup 9,gamma)-propylamine; (alpha.-2-Chloro-9-.omega.-dimethylamino-propylamine)thioxanthene; 2-Chloro-9-[.omega.-(dimethylamino)propylidene]thioxanthene; 2-Chloro-9-[3-(dimethylamino)propylidene]thioxanthene; 2-Chloro-N,N-dimethylthioxanthene-.delta.(sup 9), .gamma.-propylamine; 3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N-dimethyl-1-propanamine; 3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N-dimethylpropan-1-amine; 3-(2-chlorothioxanthen-9-ylidene)-N,N-dimethylpropan-1-amine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antipsychotic Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
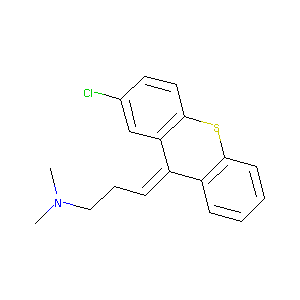 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 315.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Psychotic disorder | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A20-6A25 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
