Details of the Drug
General Information of Drug (ID: DMYBWL9)
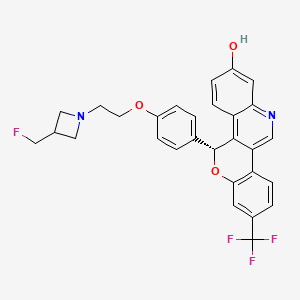
| Drug Name |
Imlunestrant
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Imlunestrant; UNII-9CXQ3PF69U; Imlunestrant [USAN]; LY3484356; 9CXQ3PF69U; 2408840-26-4; LY-3484356; WHO 12039; (5R)-5-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)phenyl)-8-(trifluoromethyl)-5H-(1)benzopyrano(4,3-c)quinolin-2-ol; 5H-(1)Benzopyrano(4,3-c)quinolin-2-ol, 5-(4-(2-(3-(fluoromethyl)-1-azetidinyl)ethoxy)phenyl)-8-(trifluoromethyl)-, (5R)-; IMLUNESTRANT [INN]; CHEMBL5095183; SCHEMBL22002569; GTPL12896; UVBQMXOKKDCBJN-MUUNZHRXSA-N; BDBM443429; GLXC-26209; US10654866, Example 1A; EX-A6123; Ly 3484356; Example 1B {WO2020014435A1]; HY-145572; CS-0376104; (5R)-5-[4-[2-[3-(fluoromethyl)azetidin-1-yl]ethoxy]phenyl]-8-(trifluoromethyl)-5H-chromeno[4,3-c]quinolin-2-ol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
