Details of the Drug
General Information of Drug (ID: DMYEALO)
| Drug Name |
TMC-647055
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
TMC647055; 1204416-97-6; TMC-647055; UNII-11BD024G7J; TMC 647055; 11BD024G7J; 113-cyclohexyl-13-methoxy-5,11-dimethyl-17H-8-oxa-4-thia-3,5,11-triaza-1(10,6)-benzo[3,4]azepino[1,2-a]indolacyclododecaphane-2,12-dione 4,4-dioxide; TMC647055 Choline; SCHEMBL1239667; CHEMBL2043025; DTXSID90152860; BDBM243456; EX-A1252; AOB87185; BCP06459; ZINC84726167; AKOS030627003; SB16907; DB11822; NCGC00482982-02; DA-47229; BC600546; 2,19-Methano-3,7:4,1-dimetheno-1H,11H-14,10,2,9,11,17-benzoxathiatetraazacyclodocosine-8,18(9H,15H)-dione, 27-cycl
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
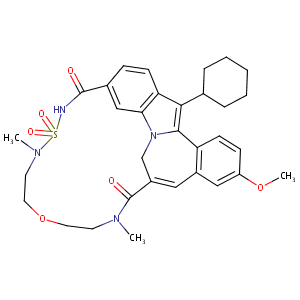 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 606.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
