Details of the Drug
General Information of Drug (ID: DMYSBN3)
| Drug Name |
AZD-9272
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
AZD 9272; 327056-26-8; UNII-54SQ9B412I; AZD9272; 54SQ9B412I; 3-fluoro-5-[3-(5-fluoropyridin-2-yl)-1,2,4-oxadiazol-5-yl]benzonitrile; CHEMBL2164550; 3-Fluoro-5-(3-(5-fluoropyridin-2-yl)-1,2,4-oxadiazol-5-yl)benzonitrile; RBSPCALDSNXWEP-UHFFFAOYSA-N; SCHEMBL2027395; GTPL6439; CHEMBL2164551; MolPort-039-338-067; ZINC33980255; BDBM50395923; AKOS027470228; HY-110254; AZD9272, > CS-0033119; J3.560.338G; 3-Fluoro-5-[3-(5-fluoro-2-pyridinyl)-1,2,4-oxadiazol-5-yl]benzonitrile
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
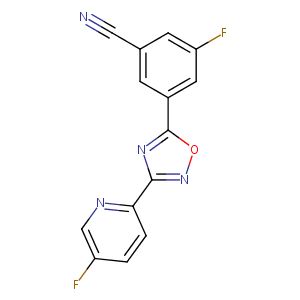 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 284.22 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Neuropathic pain | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8E43.0 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
