Details of the Drug
General Information of Drug (ID: DMZM84R)
| Drug Name |
Ethynodiol Diacetate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cervicundin; Continuin; Femulen; Luteonorm; Metrodiol; Ethinodiol diacetate; Ethyndiol Diacetate; Ethynodiol diacetate [Progestins]; Ethynodiol diacetate [USAN]; Etynodiol Acetate; Metrodiol diacetate; Ovulen 50; SC 11800; Etynodiol di(acetate); Luto-metrodiol; SC-11800; Ethynodiol diacetate (JAN/USP);[(3S,8R,9S,10R,13S,14S,17R)-17-acetyloxy-17-ethynyl-13-methyl-2,3,6,7,8,9,10,11,12,14,15,16-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl] acetate; (3 beta, 17 alpha)-19-Norpregn-4-en-20-yne-3,17 diolDiacetate; (3-beta,17-alpha)-19-Norpregn-4-en-20-yne-3,17-diol diacetate; (3beta,17beta)-17-ethynylestr-4-ene-3,17-diyl diacetate; 17-alpha-Ethynyl-19-norandrost-4-ene-3-beta,17-beta-diol diacetate; 17-alpha-Ethynyl-3,17-dihydroxy-4-estrene diacetate; 17-alpha-Ethynyl-4-estrene-3-beta,17-beta-diol diacetate; 17-alpha-Ethynylestr-4-ene-3-beta,17-beta-diol acetate; 17alpha-Ethynyl-19-norandrost-4-ene-3beta,17-beta-diol diacetate; 17alpha-Ethynyl-3,17-dihydroxy-4-estrene diacetate; 17alpha-Ethynyl-4-estrene-3beta,17beta-diol diacetate; 17alpha-Ethynylestr-4-ene-3beta,17beta-diol acetate; 17alpha-ethynylestr-4-ene-3beta,17beta-diyl diacetate; 19-Nor-17-alpha-pregn-4-en-20-yne-3-beta,17-diol diacetate; 19-Nor-17-alpha-pregn-4-en-20-yne-3-beta,17-diol, diacetate; 19-Nor-17alpha-pregn-4-en-20-yne-3beta,17-diol diacetate; 3-beta, 17-beta-Diacetoxy-17-alpha-ethynyl-4-oestrene; 3-beta,17-beta-Diacetoxy-19-nor-17-alpha-pregn-4-en-20-yne; 3beta, 17beta-Diacetoxy-17alpha-ethynyl-4-oestrene; 3beta,17beta-Diacetoxy-17alpha-ethynyl-4-estrene; 3beta,17beta-Diacetoxy-19-nor-17alpha-pregn-4-en-20-yne
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Contraceptive Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
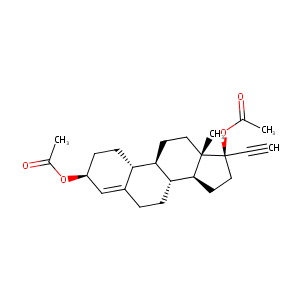 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 384.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Contraception | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | QA21 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
