| 1 |
ClinicalTrials.gov (NCT01052272) Impact of Diabetes on Left Ventricular Remodeling
|
| 2 |
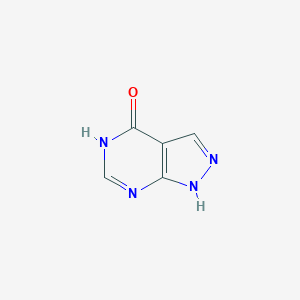
Allopurinol FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6795).
|
| 4 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 202392.
|
| 5 |
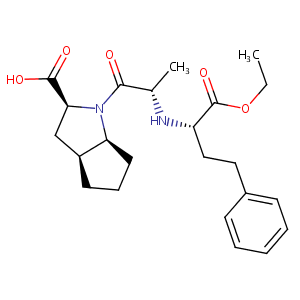
Ramipril FDA Label
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6339).
|
| 7 |
ClinicalTrials.gov (NCT04366050) Ramipril for the Treatment of COVID-19. U.S. National Institutes of Health.
|
| 8 |
Uric acid-lowering treatment with benzbromarone in patients with heart failure: a double-blind placebo-controlled crossover preliminary study. Circ Heart Fail. 2010 Jan;3(1):73-81.
|
| 9 |
Allopurinol: xanthine oxidase inhibitor. Tex Med. 1966 Jan;62(1):100-1.
|
| 10 |
Isolation, characterization and differential gene expression of multispecific organic anion transporter 2 in mice. Mol Pharmacol. 2002 Jul;62(1):7-14.
|
| 11 |
Renal transport of organic compounds mediated by mouse organic anion transporter 3 (mOat3): further substrate specificity of mOat3. Drug Metab Dispos. 2004 May;32(5):479-83.
|
| 12 |
Xanthine oxidase inhibition by allopurinol affects the reliability of urinary caffeine metabolic ratios as markers for N-acetyltransferase 2 and CYP1A2 activities. Eur J Clin Pharmacol. 1999 Jan;54(11):869-76.
|
| 13 |
Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol. 2005 May;57(5):573-8.
|
| 14 |
HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005 Mar 15;102(11):4134-9. doi: 10.1073/pnas.0409500102. Epub 2005 Mar 2.
|
| 15 |
A study of HLA class I and class II 4-digit allele level in Stevens-Johnson syndrome and toxic epidermal necrolysis. Int J Immunogenet. 2011 Aug;38(4):303-9. doi: 10.1111/j.1744-313X.2011.01011.x. Epub 2011 May 4.
|
| 16 |
Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. 2011 May;21(5):303-7. doi: 10.1097/FPC.0b013e32834282b8.
|
| 17 |
Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020 Dec;94(12):4037-4041. doi: 10.1007/s00204-020-02869-1. Epub 2020 Aug 17.
|
| 18 |
Drug-induced hepatic steatosis in absence of severe mitochondrial dysfunction in HepaRG cells: proof of multiple mechanism-based toxicity. Cell Biol Toxicol. 2021 Apr;37(2):151-175. doi: 10.1007/s10565-020-09537-1. Epub 2020 Jun 14.
|
| 19 |
Selection of drugs to test the specificity of the Tg.AC assay by screening for induction of the gadd153 promoter in vitro. Toxicol Sci. 2003 Aug;74(2):260-70. doi: 10.1093/toxsci/kfg113. Epub 2003 May 2.
|
| 20 |
Allopurinol induces innate immune responses through mitogen-activated protein kinase signaling pathways in HL-60 cells. J Appl Toxicol. 2016 Sep;36(9):1120-8. doi: 10.1002/jat.3272. Epub 2015 Dec 7.
|
| 21 |
Systemic drugs inducing non-immediate cutaneous adverse reactions and contact sensitizers evoke similar responses in THP-1 cells. J Appl Toxicol. 2015 Apr;35(4):398-406. doi: 10.1002/jat.3033. Epub 2014 Aug 4.
|
| 22 |
Allopurinol Protects Against Cholestatic Liver Injury in Mice Not Through Depletion of Uric Acid. Toxicol Sci. 2021 May 27;181(2):295-305. doi: 10.1093/toxsci/kfab034.
|
| 23 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 24 |
Clinical Pharmacogenetics Implementation Consortium guidelines for human leukocyte antigen-B genotype and allopurinol dosing. Clin Pharmacol Ther. 2013 Feb;93(2):153-8. doi: 10.1038/clpt.2012.209. Epub 2012 Oct 17.
|
| 25 |
Knockouts model the 100 best-selling drugs--will they model the next 100 Nat Rev Drug Discov. 2003 Jan;2(1):38-51.
|
| 26 |
Outcomes in Patients with COVID-19 Infection Taking ACEI/ARB. Curr Cardiol Rep. 2020 Apr 14;22(5):31.
|
| 27 |
Transport of angiotensin-converting enzyme inhibitors by H+/peptide transporters revisited. J Pharmacol Exp Ther. 2008 Nov;327(2):432-41.
|
| 28 |
Effects of an angiotensin converting enzyme inhibitor, ramipril, on intracranial circulation in healthy volunteers. off. Br J Clin Pharmacol. 1992 Sep;34(3):224-30. doi: 10.1111/j.1365-2125.1992.tb04128.x.
|
| 29 |
Ramipril inhibits in vitro human mesangial cell proliferation and platelet-derived growth factor expression. Exp Nephrol. 1999 May-Jun;7(3):229-35. doi: 10.1159/000020606.
|
| 30 |
Distinct vascular and metabolic effects of different classes of anti-hypertensive drugs. Int J Cardiol. 2010 Apr 1;140(1):73-81. doi: 10.1016/j.ijcard.2008.11.017. Epub 2008 Dec 6.
|
| 31 |
Additive beneficial cardiovascular and metabolic effects of combination therapy with ramipril and candesartan in hypertensive patients. Eur Heart J. 2007 Jun;28(12):1440-7. doi: 10.1093/eurheartj/ehm101. Epub 2007 May 5.
|
| 32 |
Contribution of human esterases to the metabolism of selected drugs of abuse. Toxicol Lett. 2015 Jan 5;232(1):159-66. doi: 10.1016/j.toxlet.2014.10.026. Epub 2014 Oct 24.
|
| 33 |
Angiotensin-converting enzyme gene polymorphism predicts the time-course of blood pressure response to angiotensin converting enzyme inhibition in the AASK trial. J Hypertens. 2007 Oct;25(10):2082-92. doi: 10.1097/HJH.0b013e3282b9720e.
|
|
|
|
|
|
|