| 1 |
ClinicalTrials.gov (NCT04087174) A Multi-centre Study to Assess the Safety, Tolerability, and Pharmacokinetics of Capivasertib (AZD5363) in Combination With Novel Agents in Patients With Metastatic Prostate Cancer
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6745).
|
| 3 |
FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 218197
|
| 4 |
ClinicalTrials.gov (NCT03997123) Capivasertib+Paclitaxel as First Line Treatment for Patients With Locally Advanced or Metastatic TNBC (CAPItello-290). U.S. National Institutes of Health.
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7709).
|
| 6 |
2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4.
|
| 7 |
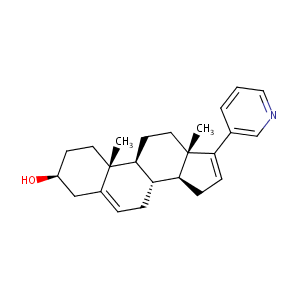
A fission yeast-based test system for the determination of IC50 values of anti-prostate tumor drugs acting on CYP21. J Enzyme Inhib Med Chem. 2006 Oct;21(5):547-56.
|
| 8 |
Elucidating mechanisms of toxicity using phenotypic data from primary human cell systems--a chemical biology approach for thrombosis-related side effects. Int J Mol Sci. 2015 Jan 5;16(1):1008-29. doi: 10.3390/ijms16011008.
|
| 9 |
Targeting CD46 for both adenocarcinoma and neuroendocrine prostate cancer. JCI Insight. 2018 Sep 6;3(17):e121497. doi: 10.1172/jci.insight.121497. eCollection 2018 Sep 6.
|
| 10 |
Targeting chromatin binding regulation of constitutively active AR variants to overcome prostate cancer resistance to endocrine-based therapies. Nucleic Acids Res. 2015 Jul 13;43(12):5880-97. doi: 10.1093/nar/gkv262. Epub 2015 Apr 23.
|
| 11 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 12 |
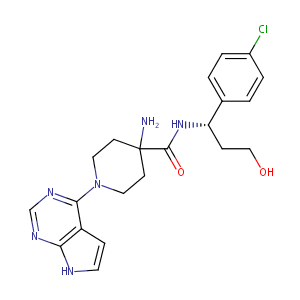
The novel AKT inhibitor afuresertib shows favorable safety, pharmacokinetics, and clinical activity in multiple myeloma. Blood. 2014 Oct 2;124(14):2190-5.
|
| 13 |
Inhibition of cholesterol metabolism underlies synergy between mTOR pathway inhibition and chloroquine in bladder cancer cells. Oncogene. 2016 Aug 25;35(34):4518-28. doi: 10.1038/onc.2015.511. Epub 2016 Feb 8.
|
|
|
|
|
|
|