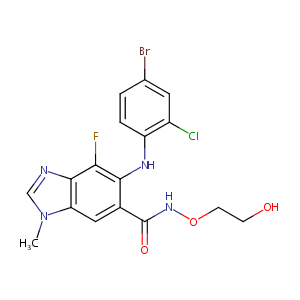
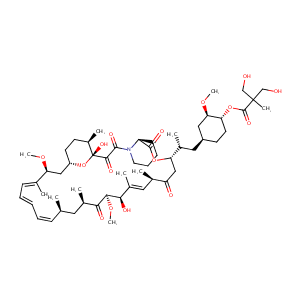
| 1 |
ClinicalTrials.gov (NCT01166126) Temsirolimus/AZD 6244 for Treatment-naive With BRAF Mutant Unresectable Stage IV
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 3 |
ClinicalTrials.gov (NCT01933932) Assess Efficacy & Safety of Selumetinib in Combination With Docetaxel in Patients Receiving 2nd Line Treatment for v-Ki-ras2 Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS) Positive NSCLC (SELECT-1)
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5665).
|
| 6 |
Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47.
|
| 7 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1479).
|
| 8 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5892).
|
| 9 |
Intrinsic resistance to selumetinib, a selective inhibitor of MEK1/2, by cAMP-dependent protein kinase A activation in human lung and colorectal cancer cells.Br J Cancer.2012 May 8;106(10):1648-59.
|
| 10 |
Antitumor action of the MET tyrosine kinase inhibitor crizotinib (PF-02341066) in gastric cancer positive for MET amplification. Mol Cancer Ther. 2012 Jul;11(7):1557-64. doi: 10.1158/1535-7163.MCT-11-0934. Epub 2012 Jun 22.
|
| 11 |
Ursonic acid exerts inhibitory effects on matrix metalloproteinases via ERK signaling pathway. Chem Biol Interact. 2020 Jan 5;315:108910. doi: 10.1016/j.cbi.2019.108910. Epub 2019 Nov 29.
|
| 12 |
The synergistic interaction of MEK and PI3K inhibitors is modulated by mTOR inhibition. Br J Cancer. 2012 Apr 10;106(8):1386-94. doi: 10.1038/bjc.2012.70. Epub 2012 Mar 13.
|
| 13 |
Advances in kinase targeting: current clinical use and clinical trials. Trends Pharmacol Sci. 2014 Nov;35(11):604-20.
|
| 14 |
Association of NR1I2, CYP3A5 and ABCB1 genetic polymorphisms with variability of temsirolimus pharmacokinetics and toxicity in patients with metastatic bladder cancer. Cancer Chemother Pharmacol. 2017 Sep;80(3):653-659.
|
| 15 |
Drug Interactions Flockhart Table
|
| 16 |
ClinicalTrials.gov (NCT00600496) A Phase I, Open-Label, Multi-center Study to Assess the Safety, Tolerability and Pharmacokinetics of AZD6244 (ARRY-142886)
|
|
|
|
|
|
|