| 1 |
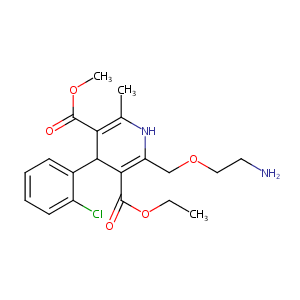
Amlodipine FDA Label
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6981).
|
| 3 |
ClinicalTrials.gov (NCT00542269) Efficacy and Safety of Aliskiren/Ramipril/Amlodipine Compared With Ramipril/Amlodipine and Aliskiren/Amlodipine in Patients With Metabolic Syndrome
|
| 4 |
ClinicalTrials.gov (NCT00402103) An Assessment of Long Term Safety of the Combination of Aliskiren / Amlodipine in Patients With High Blood Pressure
|
| 5 |
ClinicalTrials.gov (NCT00778921) Study to Evaluate the Efficacy and Safety of Combination Aliskiren/Amlodipine in Patients With Hypertension Not Adequately Responding to Amlodipine Alone
|
| 6 |
ClinicalTrials.gov (NCT00281580) Telmisartan (Micardis) and Amlodipine (Norvasc) - Factorial Design Study for the Treatment of Hypertension
|
| 7 |
ClinicalTrials.gov (NCT03626506) Spironolactone Versus Indapamide in Obese and Hypertensive Patients
|
| 8 |
ClinicalTrials.gov (NCT01354613) Reduced Contractile Reserve: a Therapeutic Target in Heart Failure With Preserved Ejection Fraction(HFpEF)
|
| 9 |
ClinicalTrials.gov (NCT00523549) The Effects of Systolic Blood Pressure Lowering on Diastolic Function Using Valsartan + Amlodipine in Patients With Hypertension and Diastolic Dysfunction
|
| 10 |
ClinicalTrials.gov (NCT00649389) Safety and Efficacy Study of a Triple Combination Therapy in Subjects With Hypertension
|
| 11 |
ClinicalTrials.gov (NCT00446563) Efficacy and Safety of Valsartan in Combination With Amlodipine Compared to Losartan Plus Hydrochlorothiazide in Patients With Hypertension and Left Ventricular Hypertrophy
|
| 12 |
ClinicalTrials.gov (NCT02933658) Study of Multiple Oral Dosing to Evaluate the Safety and the Pharmacokinetic Drug-drug Interaction of Telmisartan/Amlodipine and Rosuvastatin in Healthy Male Volunteers
|
| 13 |
ClinicalTrials.gov (NCT02789475) DP-R212 Pharmacokinetic Study
|
| 14 |
ClinicalTrials.gov (NCT01841593) A Two Way Cross Over Pharmacokinetic Interaction Study Between Raltegravir and Amlodipine in Healthy Volunteers
|
| 15 |
ClinicalTrials.gov (NCT02548286) Study to Compare the Safety and Pharmacokinetics of CKD-330 8/5mg With Coadministration of the Two Separate Drugs
|
| 16 |
ClinicalTrials.gov (NCT02121041) Ambulatory vs Office BP Management Usual Care for Diagnosing and Managing Hypertension: A Pilot Study
|
| 17 |
ClinicalTrials.gov (NCT05976438) Skin Sodium and Salt Sensitivity of Blood Pressure
|
| 18 |
ClinicalTrials.gov (NCT02152969) Drug-drug Interaction Study (Telmisartan, Amlodipine, Chlorthalidone)
|
| 19 |
ClinicalTrials.gov (NCT02474420) Amlodipine as Adjuvant Treatment to Iron Chelation for Prevention of Cardiac Iron Overload in Thalassemia Patients
|
| 20 |
ClinicalTrials.gov (NCT01549496) A Drug Interaction Study of Boceprevir in Combination With Amlodipine or Diltiazem in Healthy Volunteers
|
| 21 |
I-COMBINE study: assessment of efficacy and safety profile of irbesartan/amlodipine fixed-dose combination therapy compared with amlodipine monotherapy in hypertensive patients uncontrolled with amlodipine 5 mg monotherapy: a multicenter, phase III, prospective, randomized, open-label with blinded-end point evaluation study. Clin Ther. 2012 Aug;34(8):1705-19.
|
| 22 |
ClinicalTrials.gov (NCT00337298) The Effect of Amlodipine and Lisinopril on Retinal Autoregulation in Type 1 Diabetes
|
| 23 |
ClinicalTrials.gov (NCT01870739) A Study to Evaluate the Effect of LCZ696 on Aortic Stiffness in Subjects With Hypertension
|
| 24 |
ClinicalTrials.gov (NCT01996449) The Role of Aldosterone on Augmented Exercise Pressor Reflex in Hypertension
|
| 25 |
ClinicalTrials.gov (NCT00797862) Aliskiren and the Calcium Channel Blocker Amlodipine Combination as an Initial Treatment Strategy for Hypertension
|
| 26 |
ClinicalTrials.gov (NCT00667719) A Long Term Safety Study to Test the Combination of Aliskiren/ Amlodipine / Hydrochlorothiazide in Participants With Essential Hypertension
|
| 27 |
ClinicalTrials.gov (NCT00368277) A Safety and Efficacy Trial of Aliskiren 150mg , 300 mg Compared to Ramipril 5mg, 10mg in the Elderly
|
| 28 |
ClinicalTrials.gov (NCT01365481) Safety and Tolerability of Valsartan in Children 6 to 17 Years of Age
|
| 29 |
ClinicalTrials.gov (NCT03082014) Effects of Amlodipine and Other Blood Pressure Lowering Agents on Microvascular Function
|
| 30 |
ClinicalTrials.gov (NCT04074551) A Phase 3 Study to Compare the Efficacy and Safety of Co-administered HGP0608, HGP0904 and HCP1306 Versus HCP1701 in Patients With Hypertension and Dyslipidemia
|
| 31 |
ClinicalTrials.gov (NCT00765947) Efficacy and Tolerability of an Aliskiren-based Treatment Algorithm in Patients With Mild to Moderate Hypertension
|
| 32 |
ClinicalTrials.gov (NCT01180413) Intensive Vasodilator Therapy in Patients With Essential Hypertension
|
| 33 |
ClinicalTrials.gov (NCT01922141) Aliskiren Study of Safety and Efficacy in Senior Hypertensives
|
| 34 |
ClinicalTrials.gov (NCT01176032) ALiskiren or Losartan Effects on bioMARKers of Myocardial Remodeling
|
| 35 |
ClinicalTrials.gov (NCT02034435) Endogenous Renin-Angiotensin-Aldosterone System and Glucose Metabolism
|
| 36 |
ClinicalTrials.gov (NCT04474899) Sympathoinhibition as a Preferred Second Line Treatment of Obesity Related Hypertension
|
| 37 |
ClinicalTrials.gov (NCT03461003) N-of-1 Trials In Children With Hypertension
|
| 38 |
ClinicalTrials.gov (NCT05513937) Effectiveness and Safety of Combination of Nebivolol and Amlodipine in Hypertensive Patients Versus Each Monotherapy
|
|
|
|
|
|
|