Details of the Drug Combinations
General Information of This Drug (ID: DMJYCVW)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Brumolin; Choice; Coumadin; Coumafen; Coumafene; Coumaphen; Coumaphene; Coumarins; Coumefene; Dethmor; Dethnel; Kumader; Kumadu; Kumatox; Kypfarin; Maveran; Panwarfin; Prothromadin; RAX; Ratorex; Ratoxin; Ratron; Rattentraenke; Rattunal; Rodafarin; Rosex; Sofarin; Solfarin; Warfarat; Warfarina; Warfarine; Warfarinum; Warficide; Zoocoumarin; Arab Rat Death; Arab rat deth; Coumafene [French]; Dicusat E; Eastern states duocide; Fasco fascrat powder; Maag Rattentod Cum; Mouse pak; Ratron G; Rattenstreupulver Neu Schacht; Rattenstreupulver new schacht; Rodafarin C; Rodex blox; Sorexa plus; Temus W; Twin light rat away; Vampirinip II; Vampirinip iii; Warfarin Q; Warfarin plus; Warfarin plus [discontinued]; Zoocoumarin [Netherlands and USSR]; Zoocoumarin [Russian]; CBKinase1_000192; CBKinase1_012592; Latka 42; Latka 42 [Czech]; PS104_SUPELCO; WARF compound 42; Warf 10; Warf 42; Athrombine-K; CO-Rax; Choice (TN); Coumadin (TN); D-Con; Frass-Ratron; Jantoven (TN); Liqua-tox; Mar-Frin; Marevan (TN); Place-pax; Rac-Warfarin; Rat & mice bait; Rat-Gard; Rat-Kill; Rat-Mix; Rat-Ola; Rat-Trol; Ratten-Koederrohr; Ro-Deth; Rough & ready mouse mix; Tox-hid; Waran (TN); Warfarin (INN); Warfarin (and salts of); Warfarin [BSI:ISO]; Warfarin [INN:BAN]; Warfarin(R); Warfarina [INN-Spanish]; Warfarine [INN-French]; Warfarine [ISO-French]; Warfarinum [INN-Latin]; Cov-R-Tox; Martin's mar-frin; Rat-B-gon; Rat-a-way; Rats-no-more; Spray-trol brand roden-trol; Rat-o-cide #2; Warfarin titrated to an INR of 2.5-3.0; W.A.R.F. 42; (-)-Warfarin; (S)-Warfarin; 200 coumarin
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticoagulants
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
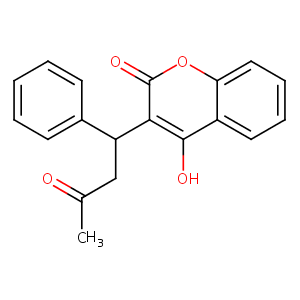 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
25 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
