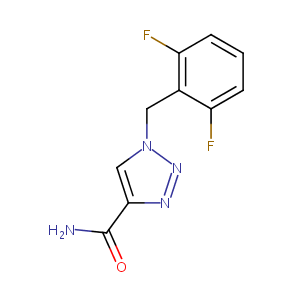
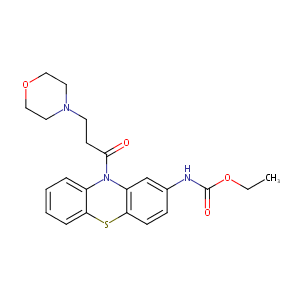
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7470).
|
| 3 |
Clinical pipeline report, company report or official report of Eisai.
|
| 4 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 5 |
Moricizine FDA Label
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7244).
|
| 7 |
Emerging drugs for epilepsy. Expert Opin Emerg Drugs. 2007 Sep;12(3):407-22.
|
| 8 |
Investigation of the metabolism of rufinamide and its interaction with valproate. Drug Metab Lett. 2011 Dec;5(4):280-9.
|
| 9 |
From first class to third class: recent upheaval in antiarrhythmic therapy--lessons from clinical trials. Am J Cardiol. 1996 Aug 29;78(4A):28-33.
|
| 10 |
Comparison of immortalized Fa2N-4 cells and human hepatocytes as in vitro models for cytochrome P450 induction. Drug Metab Dispos. 2008 Jun;36(6):1046-55.
|
|
|
|
|
|
|