Details of the Drug
General Information of Drug (ID: DMOMBJW)
| Drug Name |
Moricizine
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Moracizin; Moracizina; Moracizine; Moracizinum; Moricizine [USAN]; G 214; EN-313; Moracizina [INN-Spanish]; Moracizine (INN); Moracizinum [INN-Latin]; Moricizine (USAN); Ethyl 10-(3-morpholinopropionyl)phenothiazine-2-carbamate; Ethyl 10-(beta-N-morpholinylpropionyl)phenothiazine-2-carbamate; Ethyl N-[10-(3-morpholin-4-ylpropanoyl)phenothiazin-2-yl]carbamate; Phenothiazine-2-carbamic acid, 10-(3-morpholinopropionyl)-, ethyl ester; Ethyl [10-(3-morpholin-4-ylpropanoyl)-10H-phenothiazin-2-yl]carbamate; Ethyl {10-[3-(morpholin-4-yl)propanoyl]-10H-phenothiazin-2-yl}carbamate; Ethyl N-{10-[3-(morpholin-4-yl)propanoyl]-10H-phenothiazin-2-yl}carbamate; Ethyl (10-(3-(4-morpholinyl)-1-oxopropyl)-10H-phenothiazin-2-yl)carbamate; Carbamic acid, (10-(3-(4-morpholinyl)-1-oxopropyl)-10H-phenothiazin-2-yl)-, ethyl ester
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiarrhythmic Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
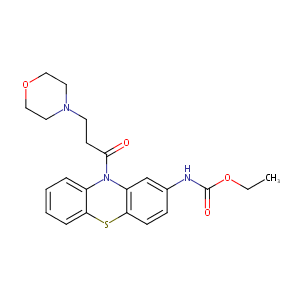 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 427.5 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Moricizine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | Moricizine FDA Label | ||||
| 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7244). | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | From first class to third class: recent upheaval in antiarrhythmic therapy--lessons from clinical trials. Am J Cardiol. 1996 Aug 29;78(4A):28-33. | ||||
| 6 | Comparison of immortalized Fa2N-4 cells and human hepatocytes as in vitro models for cytochrome P450 induction. Drug Metab Dispos. 2008 Jun;36(6):1046-55. | ||||
| 7 | Canadian Pharmacists Association. | ||||
| 8 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 9 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 10 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
