| 1 |
ClinicalTrials.gov (NCT03919292) Neratinib + Valproate in Advanced Solid Tumors, w/Expansion Cohort in Ras-Mutated Ca
|
| 2 |
Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021154)
|
| 3 |
Valproic Acid FDA Label
|
| 4 |
Immunopharmacological management of COVID-19: Potential therapeutic role of valproic acid. Med Hypotheses. 2020 May 27;143:109891.
|
| 5 |
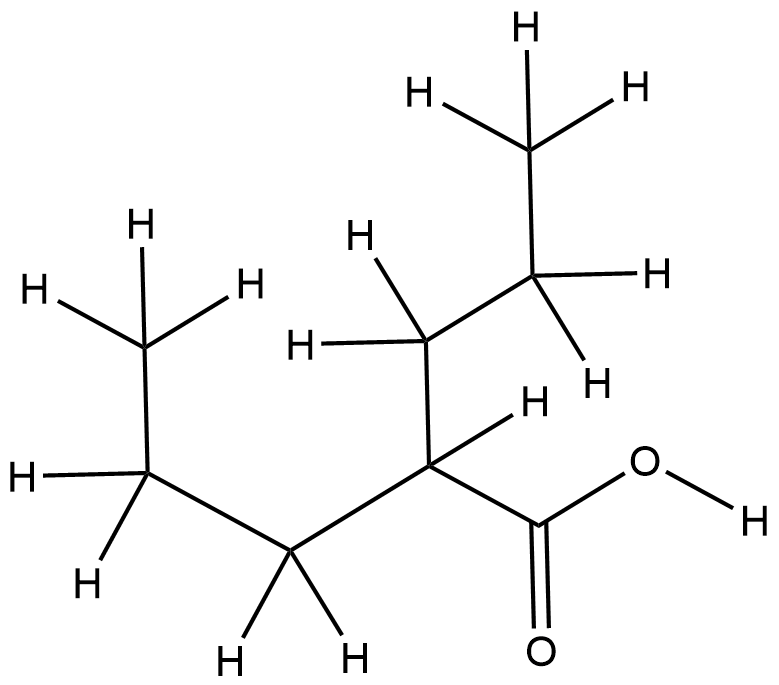
A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22.
|
| 6 |
Dual irreversible kinase inhibitors: quinazoline-based inhibitors incorporating two independent reactive centers with each targeting different cyst... Bioorg Med Chem. 2007 Jun 1;15(11):3635-48.
|
| 7 |
Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci U S A. 2007 Dec 11;104(50):19936-41.
|
| 8 |
Pharmacodynamics, pharmacokinetics and clinical efficacy of neratinib in HER2-positive breast cancer and breast cancer with HER2 mutations. Expert Opin Drug Metab Toxicol. 2016 Aug;12(8):947-57.
|
| 9 |
A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol Sci. 2013 Nov;136(1):216-41.
|
| 10 |
Irreversible EGFR inhibitor EKB-569 targets low-LET -radiation-triggered rel orchestration and potentiates cell death in squamous cell carcinoma. PLoS One. 2011;6(12):e29705. doi: 10.1371/journal.pone.0029705. Epub 2011 Dec 29.
|
| 11 |
Cysteine mapping in conformationally distinct kinase nucleotide binding sites: application to the design of selective covalent inhibitors. J Med Chem. 2011 Mar 10;54(5):1347-55. doi: 10.1021/jm101396q. Epub 2011 Feb 15.
|
|
|
|
|
|
|