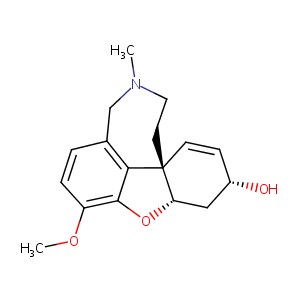
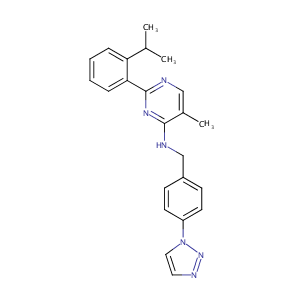
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6693).
|
| 3 |
Galantamine FDA Label
|
| 4 |
Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat Rev Drug Discov. 2018 Jan;17(1):57-78.
|
| 5 |
[From symptomatic to disease modifying therapy Recent developments in the pharmacotherapy of Alzheimer's disease]. Fortschr Neurol Psychiatr. 2009 Jun;77(6):326-33.
|
| 6 |
Clinical pharmacokinetics of galantamine. Clin Pharmacokinet. 2003;42(15):1383-92.
|
| 7 |
Lichens of parmelioid clade as promising multitarget neuroprotective agents. Chem Res Toxicol. 2019 Jun 17;32(6):1165-1177.
|
| 8 |
Inhibition of human carboxylesterases hCE1 and hiCE by cholinesterase inhibitors. Chem Biol Interact. 2013 Mar 25;203(1):226-30.
|
| 9 |
Cholinergic drugs potentiate human nicotinic alpha4beta2 acetylcholine receptors by a competitive mechanism. Eur J Pharmacol. 2005 Feb 21;509(2-3):97-108. doi: 10.1016/j.ejphar.2004.12.037.
|
| 10 |
Potencies and selectivities of inhibitors of acetylcholinesterase and its molecular forms in normal and Alzheimer's disease brain. Acta Biol Hung. 2003;54(2):183-9. doi: 10.1556/ABiol.54.2003.2.7.
|
| 11 |
Synthesis and structure-activity relationship studies of N-benzyl-2-phenylpyrimidin-4-amine derivatives as potent USP1/UAF1 deubiquitinase inhibitors with anticancer activity against nonsmall cell lung cancer. J Med Chem. 2014 Oct 9;57(19):8099-110.
|
|
|
|
|
|
|