| 1 |
ClinicalTrials.gov (NCT02530697) The Association of Miltefosine and Pentoxifylline to Treat Mucosal and Cutaneous Leishmaniasis: A Clinical Trial in Brazil
|
| 2 |
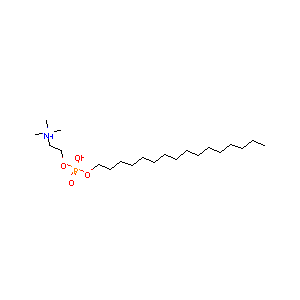
Miltefosine FDA Label
|
| 3 |
2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77-81.
|
| 4 |
Novel antifungal agents, targets or therapeutic strategies for the treatment of invasive fungal diseases: a review of the literature (2005-2009). Rev Iberoam Micol. 2009 Mar 31;26(1):15-22.
|
| 5 |
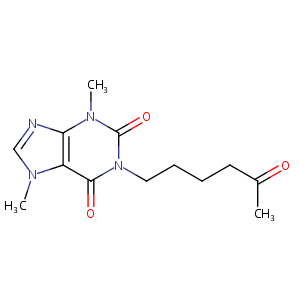
Pentoxifylline FDA Label
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7095).
|
| 7 |
Development of miltefosine as an oral treatment for leishmaniasis. Trans R Soc Trop Med Hyg. 2006 Dec;100 Suppl 1:S17-20.
|
| 8 |
Dimethylsphingosine and miltefosine induce apoptosis in lung adenocarcinoma A549?cells in a synergistic manner. Chem Biol Interact. 2019 Sep 1;310:108731. doi: 10.1016/j.cbi.2019.108731. Epub 2019 Jun 29.
|
| 9 |
MDR1 causes resistance to the antitumour drug miltefosine. Br J Cancer. 2001 May 18;84(10):1405-11. doi: 10.1054/bjoc.2001.1776.
|
| 10 |
Targeted therapies in myelodysplastic syndromes: ASH 2003 review. Semin Hematol. 2004 Apr;41(2 Suppl 4):13-20.
|
| 11 |
Cytochrome P450 isozymes involved in lisofylline metabolism to pentoxifylline in human liver microsomes. Drug Metab Dispos. 1997 Dec;25(12):1354-8.
|
| 12 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
|
|
|
|
|
|