| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 260).
|
| 3 |
Everolimus FDA Label
|
| 4 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5889).
|
| 6 |
Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47.
|
| 7 |
Emerging therapies for fibromyalgia. Expert Opin Emerg Drugs. 2008 Mar;13(1):53-62.
|
| 8 |
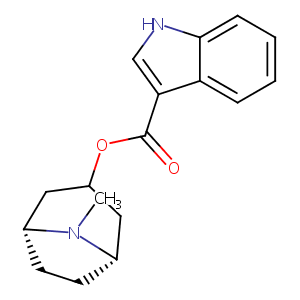
Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of dolasetron. Comparison with other indole-containing 5-HT3 antagonists. Drug Metab Dispos. 1996 May;24(5):602-9.
|
| 9 |
Cytochrome P450 2D6 metabolism and 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Med Sci Monit. 2005 Oct;11(10):RA322-8.
|
| 10 |
Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448.
|
| 11 |
Serotonin type-3 receptor antagonists selectively kill melanoma cells through classical apoptosis, microtubule depolymerisation, ERK activation, and NF-B downregulation. Cell Biol Toxicol. 2023 Jun;39(3):1119-1135. doi: 10.1007/s10565-021-09667-0. Epub 2021 Oct 15.
|
| 12 |
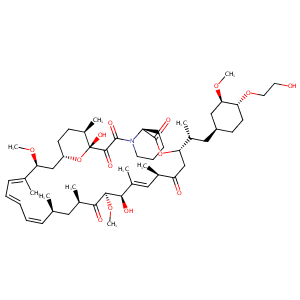
Mammalian target of rapamycin, its mode of action and clinical response in metastatic clear cell carcinoma. Gan To Kagaku Ryoho. 2009 Jul;36(7):1076-9.
|
| 13 |
Closer to the Site of Action: Everolimus Concentrations in Peripheral Blood Mononuclear Cells Correlate Well With Whole Blood Concentrations. Ther Drug Monit. 2015 Oct;37(5):675-80.
|
| 14 |
The evolving experience using everolimus in clinical transplantation. Transplant Proc. 2004 Mar;36(2 Suppl):495S-499S.
|
|
|
|
|
|
|