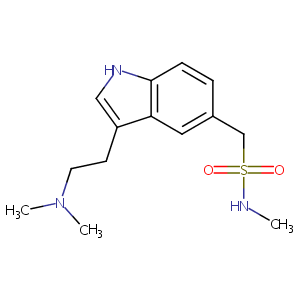
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Sumatriptan FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 54).
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 6 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 7 |
Irritable bowel syndrome: new agents targeting serotonin receptor subtypes. Drugs. 2001;61(3):317-32.
|
| 8 |
HT29-MTX and Caco-2/TC7 monolayers as predictive models for human intestinal absorption: role of the mucus layer. J Pharm Sci. 2001 Oct;90(10):1608-19. Comparative Study
|
| 9 |
Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42.
|
| 10 |
A toxicogenomic approach to drug-induced phospholipidosis: analysis of its induction mechanism and establishment of a novel in vitro screening system. Toxicol Sci. 2005 Feb;83(2):282-92.
|
| 11 |
The effects of oral sumatriptan, a 5-HT1 receptor agonist, on circulating ACTH and cortisol concentrations in man. Br J Clin Pharmacol. 1995 Apr;39(4):389-95. doi: 10.1111/j.1365-2125.1995.tb04467.x.
|
| 12 |
G protein beta3 polymorphism and triptan response in cluster headache. Clin Pharmacol Ther. 2007 Oct;82(4):396-401. doi: 10.1038/sj.clpt.6100159. Epub 2007 Mar 14.
|
| 13 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 14 |
Sivelestat (selective neutrophil elastase inhibitor) improves the mortality rate of sepsis associated with both acute respiratory distress syndrome... Shock. 2010 Jan;33(1):14-8.
|
| 15 |
Neutrophil elastase inhibitor (sivelestat) reduces the levels of inflammatory mediators by inhibiting NF-kB. Inflamm Res. 2009 Apr;58(4):198-203.
|
|
|
|
|
|
|