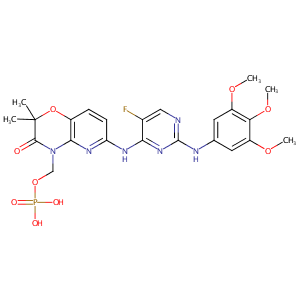
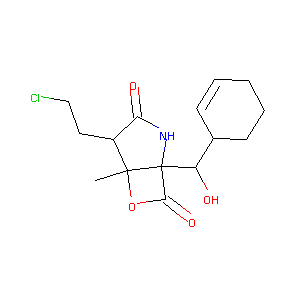
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89.
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7796).
|
| 4 |
ClinicalTrials.gov (NCT03345095) A Phase III Trial of With Marizomib in Patients With Newly Diagnosed Glioblastoma (MIRAGE). U.S. National Institutes of Health.
|
| 5 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 6 |
Clinical pipeline report, company report or official report of Triphase Accelerator .
|
| 7 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2230).
|
| 8 |
DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018 Jan 4;46(D1):D1074-D1082. (ID: DB12010)
|
| 9 |
Effects of CYP3A4 inhibitors ketoconazole and verapamil and the CYP3A4 inducer rifampicin on the pharmacokinetic parameters of fostamatinib: results from In vitro and phase I clinical studies. Drugs R D. 2016 Mar;16(1):81-92.
|
| 10 |
Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011 Mar;11(3):254-84.
|
|
|
|
|
|
|