| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 3 |
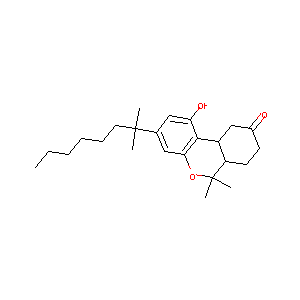
Novel 1',1'-chain substituted hexahydrocannabinols: 9-hydroxy-3-(1-hexyl-cyclobut-1-yl)-hexahydrocannabinol (AM2389) a highly potent cannabinoid r... J Med Chem. 2010 Oct 14;53(19):6996-7010.
|
| 4 |
Cloning and pharmacological characterization of the dog cannabinoid CB?receptor. Eur J Pharmacol. 2011 Nov 1;669(1-3):24-31. doi: 10.1016/j.ejphar.2011.08.002. Epub 2011 Aug 19.
|
| 5 |
The fight against drug-resistant malaria: novel plasmodial targets and antimalarial drugs. Curr Med Chem. 2008;15(2):161-71.
|
| 6 |
Development of a paediatric physiologically based pharmacokinetic model to assess the impact of drug-drug interactions in tuberculosis co-infected malaria subjects: A case study with artemether-lumefantrine and the CYP3A4-inducer rifampicin. Eur J Pharm Sci. 2017 Aug 30;106:20-33.
|
|
|
|
|
|
|