| 1 |
ClinicalTrials.gov (NCT04373564) Effect on Body Movement and Mental Skills in Patients Who Received Gadolinium-based Contrast Media for Magnetic Resonance Examination Multiple Times Within 5 Years
|
| 2 |
2004 approvals: the demise of the blockbuster. Nat Rev Drug Discov. 2005 Feb;4(2):93-4.
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
Protocol design for high relaxivity contrast agents in MR imaging of the CNS. Eur Radiol. 2006 Nov;16 Suppl 7:M3-7.
|
| 5 |
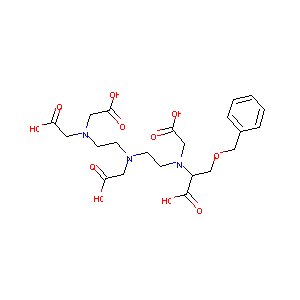
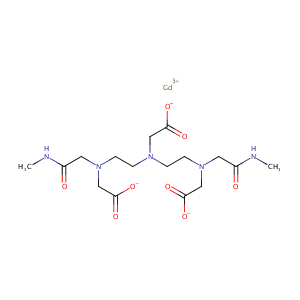
Liver Perfusion Modifies Gd-DTPA and Gd-BOPTA Hepatocyte Concentrations Through Transfer Clearances Across Sinusoidal Membranes. Eur J Drug Metab Pharmacokinet. 2017 Aug;42(4):657-667.
|
|
|
|
|
|
|