Details of the Drug Combination
General Information of Drug Combination (ID: DCJFU5F)
| Drug Combination Name |
Ruxolitinib Enasidenib
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication |
|
|||||||||||||||||
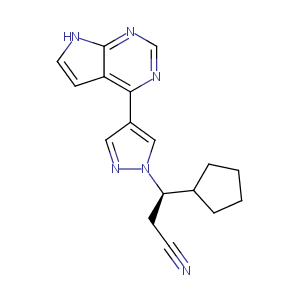
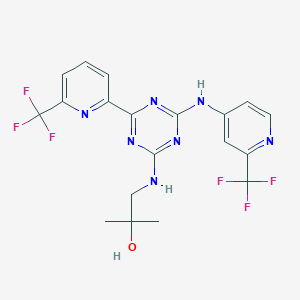
| Component Drugs | Ruxolitinib | Enasidenib | ||||||||||||||||
| Small molecular drug | N.A. | |||||||||||||||||
|
|
|||||||||||||||||
| 2D MOL | 2D MOL | |||||||||||||||||
| 3D MOL | 3D MOL | |||||||||||||||||
Molecular Interaction Atlas of This Drug Combination
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication(s) of Ruxolitinib |
|
|||||||||||||||||||||||||||||||||||||||||||||||
|
Ruxolitinib Interacts with 5 DTT Molecule(s)
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Ruxolitinib Interacts with 1 DOT Molecule(s)
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Enasidenib Interacts with 5 DME Molecule(s)
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Enasidenib Interacts with 1 DOT Molecule(s)
|
||||||||||||||||||||||||||||||||||||||||||||||||
References
