Details of the Drug
General Information of Drug (ID: DM8QXOC)
| Drug Name |
Enasidenib
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Enasidenib; Enasidenib mesylate; IDHIFA; 1446502-11-9; 2-Methyl-1-(4-(6-(trifluoromethyl)pyridin-2-yl)-6-(2-(trifluoromethyl)pyridin-4-ylamino)-1,3,5-triazin-2-ylamino)propan-2-ol; 2-Propanol, 2-methyl-1-((4-(6-(trifluoromethyl)-2-pyridinyl)-6-((2-(trifluoromethyl)-4-pyridinyl)amino)-1,3,5-triazin-2-yl)amino)-; 2-methyl-1-((4-(6-(trifluoromethyl)pyridin-2-yl)-6-((2-(trifluoromethyl)pyridin-4-yl)amino)-1,3,5-triazin-2-yl)amino)propan-2-ol; 3T1SS4E7AG; AG-221; CC-90007; CC-90007 Free Base; UNII-3T1SS4E7AG
|
|||||
| ATC Code | ||||||
| Structure |
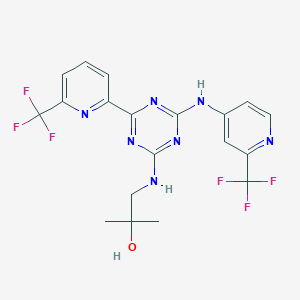 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 473.4 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 3.5 | |||||
| Rotatable Bond Count (rotbonds) | 6 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 14 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
References
