| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
ClinicalTrials.gov (NCT01126073) A Double Blind, Randomized Study to Compare Influence of Niacin/Laropiprant on Functional and Morphological Characteristics of Arterial Wall and Parameters of Inflammation in Subjects With Coronary Heart Disease Already Treated With a Statin in Miran Sebestjen, University Medical Centre Ljubljana.
|
| 3 |
Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800023729)
|
| 4 |
ClinicalTrials.gov (NCT03345095) A Phase III Trial of With Marizomib in Patients With Newly Diagnosed Glioblastoma (MIRAGE). U.S. National Institutes of Health.
|
| 5 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 6 |
Clinical pipeline report, company report or official report of Triphase Accelerator .
|
| 7 |
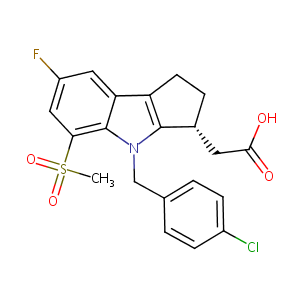
Metabolism of MK-0524, a prostaglandin D2 receptor 1 antagonist, in microsomes and hepatocytes from preclinical species and humans. Drug Metab Dispos. 2007 Feb;35(2):283-92. doi: 10.1124/dmd.106.011551. Epub 2006 Nov 28.
|
| 8 |
Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydroc... J Med Chem. 2007 Feb 22;50(4):794-806.
|
| 9 |
A novel c-Met inhibitor, MK8033, synergizes with carboplatin plus paclitaxel to inhibit ovarian cancer cell growth. Oncol Rep. 2013 May;29(5):2011-8.
|
| 10 |
Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011 Mar;11(3):254-84.
|
|
|
|
|
|
|