| 1 |
ClinicalTrials.gov (NCT03970330) Low-Dose Naltrexone in Combination With Standard Treatment in Women With Endometriosis
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 3 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 074918.
|
| 4 |
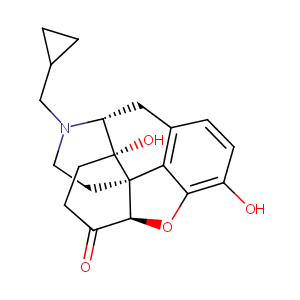
Naltrexone FDA Label
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1639).
|
| 6 |
ClinicalTrials.gov (NCT04365985) Study of Immunomodulation Using Naltrexone and Ketamine for COVID-19. U.S. National Institutes of Health.
|
| 7 |
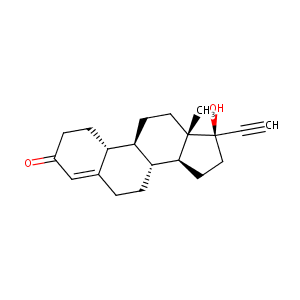
Norethindrone FDA Label
|
| 8 |
An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008 Feb;65(2):135-44.
|
| 9 |
In vivo chronic exposure to heroin or naltrexone selectively inhibits liver microsome formation of estradiol-3-glucuronide in the rat. Biochem Pharmacol. 2008 Sep 1;76(5):672-9.
|
| 10 |
Low dose naltrexone therapy in multiple sclerosis. Med Hypotheses. 2005;64(4):721-4.
|
| 11 |
Chronic naltrexone treatment induces desensitization of the luteinizing hormone pulse generator for opioid blockade in hyperprolactinemic patients. J Clin Endocrinol Metab. 1995 May;80(5):1739-42. doi: 10.1210/jcem.80.5.7745028.
|
| 12 |
Association between the Stin2 VNTR polymorphism of the serotonin transporter gene and treatment outcome in alcohol-dependent patients. Alcohol Alcohol. 2008 Sep-Oct;43(5):516-22. doi: 10.1093/alcalc/agn048. Epub 2008 Jun 14.
|
| 13 |
Predicting the effect of naltrexone and acamprosate in alcohol-dependent patients using genetic indicators. Addict Biol. 2009 Jul;14(3):328-37.
|
| 14 |
Effects of 17beta-estradiol, progesterone, synthetic progestins, tibolone, and raloxifene on vascular endothelial growth factor and Thrombospondin-1 messenger RNA in breast cancer cells. Int J Gynecol Cancer. 2006;16 Suppl 2:560-3. doi: 10.1111/j.1525-1438.2006.00696.x.
|
| 15 |
Progesterone receptor ligand binding pocket flexibility: crystal structures of the norethindrone and mometasone furoate complexes. J Med Chem. 2004 Jun 17;47(13):3381-7.
|
| 16 |
P-glycoprotein (P-gp/MDR1)-mediated efflux of sex-steroid hormones and modulation of P-gp expression in vitro. Pharm Res. 2004 Jul;21(7):1284-93.
|
| 17 |
Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 2010 Dec; 118(2):485-500.
|
| 18 |
Progestins as inhibitors of the human 20-ketosteroid reductases, AKR1C1 and AKR1C3. Chem Biol Interact. 2011 May 30;191(1-3):227-33.
|
| 19 |
A Gene Expression Biomarker Identifies Chemical Modulators of Estrogen Receptor in an MCF-7 Microarray Compendium. Chem Res Toxicol. 2021 Feb 15;34(2):313-329. doi: 10.1021/acs.chemrestox.0c00243. Epub 2021 Jan 6.
|
| 20 |
Picomolar norethindrone in vitro stimulates the cell proliferation and activity of a human osteosarcoma cell line and increases bone collagen synthesis without an effect on bone resorption. J Bone Miner Res. 1994 May;9(5):695-703. doi: 10.1002/jbmr.5650090515.
|
| 21 |
Pharmacologic reductions of total tau levels; implications for the role of microtubule dynamics in regulating tau expression. Mol Neurodegener. 2006 Jul 26;1:6. doi: 10.1186/1750-1326-1-6.
|
| 22 |
Novel genotoxicity assays identify norethindrone to activate p53 and phosphorylate H2AX. Carcinogenesis. 2005 Oct;26(10):1811-20. doi: 10.1093/carcin/bgi132. Epub 2005 May 19.
|
| 23 |
The estrogenic activity of synthetic progestins used in oral contraceptives enhances fatty acid synthase-dependent breast cancer cell proliferation and survival. Int J Oncol. 2005 Jun;26(6):1507-15.
|
|
|
|
|
|
|